


 |
Teaching : A Lifetime of Learning Milton Orchin Birthday Celebration, December 10, 1999 |
|
Introduction to this document, taken from
On December 10, 1999, the Chemistry Department held the
"85.5th Birthday Party" in honor of Professor Milton Orchin.
For those of us privileged to attend, this was an unforgettable experience.
 From The Editor {John. S. Thayer, Ph.D., website note} One highlight was Professor Orchin‘s address Teaching: A Lifetime of Learning , which we are proud to reproduce, slightly edited, in this special tribute issue. At that party, a memorial booklet entitled What a Privilege! was issued to the attendees. Some copies of this booklet, which commemorates Professor Orchin‘s career, are still available; if you would like a copy, please send a note or FAX :Please address all correspondence to here. Among Professor Orchin‘s many accomplishments was the establishment of the Departmental Newsletter, now known as CHEM BOND. .... .... We wish to express our sincere and deepfelt thanks to Kim Carey {Editorial Assistant, website note} for the time and effort she has put in, both on the birthday party and on this special issue. News Flash : We have just {Fall 2000, website note} learned that Professor Orchin has been included in the recent book Candid Science written by Istvan Hargittai (Editor of the journal Chemical Intelligencer). Only thirty-six chemists from around the world have been included, and these include eighteen Nobel Laureates! In Professor Orchin‘s own words, "I feel pretty humbled to be included in the prestigious 36. lt is the most important recognition that I have ever received, and I owe a lot to the University of Cincinnati and the Department of Chemistry for the opportunities which made this recognition possible." |
I want to thank all of you for being here and especial thanks to those of you who have traveled from cities around the country to share with me and the Department of Chemistry what is for me and my loved ones a memorable occasion. This is, as you know, a kind of birthday celebration. Although I won‘t dwell on the significance and good fortune of exceeding the biblical lifetime span of three score and ten, I never thought reaching 85 would be something that I would personally experience but rather something that I would read about others experiencing. For those of you who have never met my immediate family and for those of you who have and wish to revive memories of them I would like to introduce them now starting with my youngest son ... .... .... I did my undergraduate studies at OSU in Columbus. I didn‘t have a problem agonizing over school choice because the in-state tuition for a full year there at that time was $60.00. For me OSU was ideal. It was a big school with many distinguished faculty and I liked the anonymity. I appreciated the quarter system, for it allowed a large choice of subjects outside the major. I was unbelievably fortunate in selecting Mel Newman as my graduate school mentor, and lucky to spend my first year of graduate studies working in the intimacy of the small laboratory I shared with him as his first graduate student. As many of you know, he was a master of laboratory techniques. He taught me what it means to be efficient in the lab; not to do anything more than was necessary but to take great care and patience with what could be significant. His publications reflected bis thinking: “Get to the point and eliminate every unnecessary word!“ His enthusiasm was contagious and he instilled in me a lifetime love of chemistry. When I think of Mel‘s influence I am reminded of an admonition that Albert Einstein gave: “Everything should be made as simple as possible but not simpler‘. For ten years prior to corning to the University of Cincinnati, I worked at the U.S. Bureau of Mines in the Synthetic Fuels Division. The major goal of this work was to promote the commercialization of processes which convert coal (a solid fuel) to a liquid transportation fuel resembling petroleum. And in studying various aspects of coal chemistry, I began to understand the importance of catalysis and developed a life-time interest in the subject. One ofthe aspects of catalysis that has always appealed to me is that it is a way to save time. Time is, as it should be, the most precious human possession. Time is what is important to us all, because we know our own time is limited and we strive to make the most of what is dealt to us. The ultimate goal of catalysis is to make desirable things in a shorter period of tirne and with less expenditure of energy. |
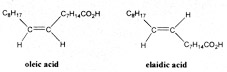 |
Now oleic acid is a long straight chain of carbon atoms terminated by a carboxyl group, right in the middle of the chain is a double bond which makes it a mono-unsaturated acid. The two halves of the chain in oleic acid are on the same side of this double bond. Such an arrangement is called a cis form. |
| When finally he got the equipment to work, the distilled material started to come
over through a cold condenser. Well, we both expected the material to solidify on cooling but
instead it came over as a liquid! The meaning of this was devastatingly clear. After all the work
to prepare the high melting trans acid, it was being converted on distillation back into the liquid
cis acid! |
Editor‘s Note: The biochemistry of selenium is a very active field of current research. Selenium apparently has several functions. The amino acid selenocysteine can be incorporated into proteins and occurs in the antioxidant enzyme glutathione peroxidase. which controls H-,O, activity in aerobic cells [Wolinsky & Driskell. ed. Sports Nutrition. CRC Press, 1997, pp 195-204]. Selenium and Vitamin E serve as synergistic antioxidants. The ability of selenium to undergo electron transfer may be a crucial part of its biological function.
