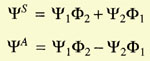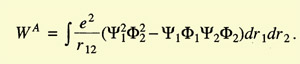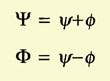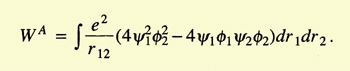QUANTUM CHEMISTRY is usually regarded as a success story of quantum
mechanics. The community of physicists, in any case, came under the spell
of Dirac's reductionist program, expressed as a theoretically correct, but
practically meaningless dictum
(1) :
 P.A.M. Dirac
P.A.M. Dirac
1902-1984
Photo from
Nobel Foundation.
|
The general theory of quantum mechanics is now almost
complete, the
imperfections that still remain being in connection with the exact
fitting of the theory with relativity ideas ...
The underlying physical laws necessary for the
mathematical theory of a large part of physics and the whole of
chemistry are thus completely known, and the difficulty is only
that the exact application of
these laws leads to equations much too complicated to be
soluble.
It appeared theoretical chemistry amounted to no more than quantum
mechanical calculation.
We argue that the development of quantum chemistry involved issues
that transcended the application of quantum mechanics to chemical problems.
Quantum chemistry developed an autonomous language. What
appeared to be disputes over computational details were discussions about
the collective decision of the chemical community on methodological
priorities and ontological commitments. The outstanding issue turned out to be
the character of theory for chemistry and, therefore, a reappraisal of the
praxis of chemists.
|
Nearly all books and research papers consider two approaches to atomic
bonding: the Heitler-London-Pauling-Slater (H-L-P-S) method of valence
bonds and the Hund-Mulliken (H-M) method of molecular orbitals. We
argue for a different conception : Heitler-London versus Mulliken-Pauling
{set in italic by this website}.
We regard as the dominant criterion the views of the protagonists about
theory building and the role of theory for chemistry. Heitler and London
insisted on an approach close to Dirac's reductionist pronouncement of
1929. Pauling and Mulliken had a strong inclination toward semi-empirical
methods. This characterization does not imply that either Heitler and
London, or Mulliken and Pauling, agreed on everything. Heitler proved in the
end to be comfortable with a much more reductionist approach than London.
The disagreement between Mulliken and Pauling over valence theory
concerned questions for more fundamental than computational details.
The differences between the two approaches (H-L or M-P) can be
understood in terms of two different cultures. To some Germans (at least to
Heitler and London), American pragmatism appeared flippant. To some
Americans (at least to Mulliken, Pauling, Slater, and Van Vleck), the German
mania to do everything from first principles appeared as unnecessarily
cumbersome and tortuous. At times there appeared to be a confluence of
different styles of research and at other times an uneasy feeling that not all
differences could be reconciled.
The wonderful matchmaker: G.N. Lewis and his pairing of electrons
In 1916, Gilbert Newton Lewis proposed that chemical bonding - both
the ionic and the homopolar type - could be explained in terms of shared
electron pairs. He supposed that each atom in a molecule in most cases had
eight electrons in outer orbits, and that in homopolar bonds the "rule of
eight" could be realized by the sharing of pairs of electrons between
atoms.
"In my paper on ' The Atom and the Molecule ', I laid much
stress upon the
phenomenon of pairing of electrons. I have since become convinced that
this phenomenon is of even greater significance than I then
supposed... .There is nothing in the known laws of electric force, nor is
there anything in the quantum theory of atomic structure, as far as it has yet
been developed, to account for such a pairing."
(2)
 J.J. Thomson
J.J. Thomson
1856-1940
Photo originating from here.
|
Before 1916, the possibility of explaining homopolar bonding by "sharing"
or "exchanging" one or two electrons had occurred to several people.
In 1914, J.J. Thomson proposed a model in which the stability of an atom
could be guaranteed if the electrical forces between the nucleus and the
orbiting electrons were confined to narrow tubes.
(3) Extending these
ideas, he
made polar and non-polar bonds two distinct types. Polar bonds were realized
through the transfer of electrons, non-polar bonds via a tube of force
connecting an electron of one atom with the nucleus of another. Also in
1914, William Arsem proposed that the non-polar bond consisted in the
sharing of one electron. Hence the electrically neutral units of matter were
the molecules and the electronic charge should have been twice the value
used for the ionic mechanism.
|
|
(4)
In 1915 Alfred Parson published a paper
exploring the possibilities provided by magnetism for the formation of
molecules.
(5)
He proposed a model of the atom with magnetons arranged at
the corners of a cube. Each magneton was a circular band of electricity in
rapid revolution; chemical affinity arose from the magnetic moments
generated by the motions. In 1916 Walther Kossel put forth a number of
proposals about valence, insisting on the "rule of eight."
(6)
He did not commit
himself on the crucial point of the shared electron pair; the mechanism he
proposed for chemical bonding depended on electron transfer. He made
one exception: a special non-polar bond created by rings of two to five
electrons with orbits perpendicular to the bond axis.
Lewis thought that Bohr's theory provided the theoretical basis of his
ideas for the chemical bond. But he regarded the orbit as a whole, rather
than as a succession of positions of an electron within the orbit, as the
essential aspect of Bohr's theory. "If these orbits are in fixed positions and
orientations they may be used as the building stones of an atom which has
an essentially static character."
(7)
|
|
 Walther Kossel
Walther Kossel
(1888-1956)
Photo from here. |
Assigning an independent orbit to each
electron, considering it as having a fixed position in space, and identifying
the fixed position of the electrons in the static atom with the average
position of the electron in the orbit, reconciled the physicists' atom with the
chemists' atom. Furthermore, this interpretation of Bohr provided a
theoretical justification for the notion of shared pairs and, by making the
notion less dependent on the "cubic" model, it anchored Lewis's theory of
the chemical bond more securely than the octet rule.
Lewis's reconciliation of the two views rested not only on his ontological
commitments ("it is the same atom and the same molecule that is being
studied by the organic chemist, inorganic chemist and physicist),"
(8)
but also
on the hope that the sharing of the electron pairs could unify the two
disparate theories of the chemical bond. "The suggestion of two entirely
distinct kinds of chemical union, one for polar and the other for non-polar
compounds was repugnant to the chemical instinct which leads so irresistibly
to the belief that all types of chemical union are essentially one and the
same."
(9)
But such an attitude implied the sacrifice of the notion of the
discreteness of the electric charge. He wrote to A.A. Noyes that he could
no longer "subscribe to [your] fundamental idea that an atom must possess
an integral muItiple of the elementary charge."
(10)
This was Lewis's view in 1916. The purpose of his work on valence
was to show how it would be possible to obtain a complete continuity
between extremely polar and extremely non-polar compounds by giving up
the idea of discreteness. As he told Noyes, he intended to substitute for it
the "idea of shared electrons which could range all the way between
complete possession by one atom, which corresponds to what you still call the
polar bond, and the most equitable division between two atoms which
would correspond with your idea that the charge of an electron pair is
shared equally by the two bonded atoms."
In 1923, soon after the publication of Lewis' Valence and the structure
of atoms and molecules, the Faraday Society organized a meeting at the
University of Cambridge. The theme of the meeting was the "Electronic
theory of valency." In the opening address, J.J. Thomson said that "The
bond dominates the field of chemistry, which finds its most suggestive
mode of expression in terms of electrons. Admitting the presence of electrons,
their repulsion involves important chemical consequences."
(11)
Lewis gave the introductory talk. He declared that the two views of the
atom derived from the study of chemistry and physics were "completely
reconcilable." The "cardinal phenomenon of all chemistry" was the
formation of electron pairs, actual physical pairings; eventually quantum
theory would explain the pairing. Lewis insisted that the two kinds of
bonds, polar and non-polar, could be interpreted in terms of the relative
position of the electron pair with respect to the nuclei of the molecule and,
hence, that there was fundamentally but a single mechanism for chemical
bonding.
|
 G.N. Lewis
G.N. Lewis
1875–1946
Photo from
chemheritage.org
|
While discussing the problem of multiple bonds, Lewis expressed opinions
that would be incorporated in the resonance approach introduced by
Pauling in the early 1930s. Where more than one electron pairs exist, they
cannot be on the line joining the two atomic centers. There arose a very
serious problem of lack of definiteness, "which is to be ascribed, not to our
modes of expression, but to the nature of the actual structure of the
molecule." How far can an electron pair be removed from the line joining
the atomic centers and still be regarded as belonging to both atoms? Lewis
had no second thoughts about the implicit arbitrariness of an answer to such
a question: one should consider all the possible arrangements and the actual
state of affairs lies "somewhere between" these possible structures.
By 1927, most chemists had become aware of the amazing explanatory
power of the new quantum mechanics, but they did not see how to assimilate
it. Moreover assimilation might bring lasting changes to their
disciplinary cuIture. It was, nevertheless, a risk worth taking. N.V. Sidgwick,
in his influential book The electronic theory of valency,
would have no inhibitions about letting the new quantum mechanics invade the realm
of chemistry. Faced with the full development of the new mechanics by Heisenberg
and Schrödinger, but not with an application of the theory to a chemical
problem, Sidgwick attempted to clarify the methodological stumbling block
in the way of his fellow chemists :
(12)
In developing a theory of valency there are two courses open to the chemist.
He may use symbols with no definite physical connotation to express the
reactivity of the atoms in a molecule, and may leave it to the subsequent
progress of science to discover what realities these symbols represent:
or he may
adopt the concepts of atomic physics - electrons, nuclei, and orbits - and
try to explain the chemical facts in terms of these.
But if he takes the latter course,
as is done in this book, he must accept the physical conclusions in full, and
must not assign to these entities properties which the physicists have found
them not to possess: he must not use the terminology of physics unless he is
prepared to recognize its laws. I have endeavored to conform to this
principle, and not to lay myself open to the reproach of an eminent physicist,
that
"when chemists talk about electrons they use a different language from the
physicists." I have been careful to avoid as far as possible the introduction
of any physical hypotheses which are not already sanctioned by those who
are best qualified to judge of them.
The young Mulliken
In the year that Lewis's Valence appeared, E.C. Kemble and
R.T. Birge,
together with W.F. Colby, F.W. Loomis, and L. Page, started preparing a
comprehensive report under the auspices of the National Research Council
on the spectra of molecular diatomic gases.
(13)
The molecular spectroscopists
assumed three different contributions: rotational, vibrational, and electronic.
An increasingly sophisticated model of the rotational and vibrational
nuclear motions of diatomic molecules guided them through the maze of
band spectra to a detailed knowledge of molecular structure.
(14)
In 1921, R.S. Mulliken completed his Ph.D. at the University of Chicago
working on the separation of isotopes with the physical chemist W.D.
Harkens. He stayed in Chicago one more year as a National Research
Council postdoctoral fellow and then moved to Harvard. There he worked
on molecular spectroscopy with F.A. Saunders and Kemble and later helped
prepare the report to the National Research Council on the spectra of
diatomic molecules.
|
Isotope effects in the spectra of diatomic molecules aroused Mulliken's
interest in the electronic distribution in molecules.
(15) By 1925
several electronic levels had been identified in very simple molecules and
molecular
fragments: CO (five electronic levels), N2 and NO (four levels),
and BO,
CN, CO+, and 02 (three levels).
(16)
As their number increased, so did the
need for classification. Spectroscopists searched for analogies in the
spectroscopic behavior of different compounds. Following earlier suggestions
on the similarities between certain molecular and atomic spectra and on the
physical similarities of isosteric molecules (compounds with the same
number of elements and the same total number of electrons), Mulliken
looked for similarities in the spectra of isosteric molecules.
(17)
He found that
the spectroscopic analogy between isosteric molecules could be extended to
the chemical element with the same number of electrons.
The parallels between molecular and atomic spectra served as the basis
for the classification of diatomic molecules into different families and
suggested that similar electronic distributions were responsible for corresponding
systems of energy levels.
|
|

R.S. Mulliken, 1929
1896-1986
Photo from
R.S. Mulliken,
Life of a Scientist,
Springer 1989.
|
Although "pretty speculative",
(18)
the analogy
had been used by several 'scientists, including R. Mecke and
H. Sponer in
Germany and Birge in the United States.
(19)
It became a recurring theme in
the extensive correspondence between Birge and Mulliken.
(20)
Together with evidence that the electronic levels of CO,
NFR2, and H2
approximately fit
the formulas for line spectra (such as the Rydberg or Ritz formulas), these
analogies led Birge to postulate that "the energy levels associated with the
valence electrons of molecules agree in all essential aspects with those
associated with the valence electrons of atoms."
(21)

Hertha Sponer
1895-1968
Austin 1955
|
With Birge's proposal one could classify electronic states in diatomic
molecules by means of the same nomenclature (Russel-Saunders notation)
used for atomic states (2S, 2P, 3S,
1S, 1P). Mulliken immediately looked for
corroborative evidence.
(22)
Going one step further, he introduced three postulates that accounted for
known band spectra and predicted yet unanalyzed
band spectra.
(23)
Mulliken soon addressed the question of molecule formation
and for the first time hinted at what he would later call "electron promotion",
a concept essential to his theory of chemical binding: in the formation
of molecules a radical rearrangement of some electrons may take place,
corresponding to their "promotion" to orbits with a higher n quantum
number.
(24)
|
With Birge's proposal one could classify electronic states in diatomic
molecules by means of the same nomenclature (Russel-Saunders notation)
used for atomic states (2S, 2P, 3S,
1S, 1P). Mulliken immediately looked for
corroborative evidence.
(22)
Going one step further, he introduced three postulates that accounted for
known band spectra and predicted yet unanalyzed
band spectra.
(23)
Mulliken soon addressed the question of molecule formation
and for the first time hinted at what he would later call "electron promotion",
a concept essential to his theory of chemical binding: in the formation
of molecules a radical rearrangement of some electrons may take place,
corresponding to their "promotion" to orbits with a higher n quantum
number.
(24)

F. Hund 1929
1896-1997
Photo taken from here.
|
When Mulliken read Friedrich Hund's theoretical discussion of the
nature of the electronic states,
(25)
which introduced electron spin into band
structure, he immediately recognized its importance and excitedly confided
to Birge:
(26)
Hund really has everything in his paper. It's most
remarkable. Nature of
electronic states and fine structure both although the experimental evidence
at
the time was rather small (and he didn't discuss it very carefully). But
almost all of my conclusions seem to agree with his theory. Of course it may
not all be right. Dr. Sommer (of Göttingen) says Hund is going right into
molecule spectra now.
Mulliken published a summary of Hund's theory and provided an extensive
discussion of the empirical evidence, which relied heavily on his own
previous work.
(27)
Hund's quantum mechanical approach to molecules was largely
confirmed by Mulliken's systemization of band spectra, and Mulliken's
phenomenological theory found a legitimizing framework.
|
In the summer of 1927 Mulliken went to Europe. He visited Göttingen,
Zürich, and Geneva, and ended the summer with a hiking trip to the Black
Forest with Hund and some friends. He wanted to discuss with Hund his
new contributions to a quantum mechanical theory of molecular structure.
Contrary to Hund, Mulliken's attitude toward the new quantum mechanics
was pragmatic; "[I] was satisfied with a general knowledge of [quantum
mechanical] methods and principles sufficient to help me understand particular
molecules or types of molecules and their properties; especially their
spectra. In short, I was more interested in getting better acquainted with
molecules than with abstract theory about them.
(28)
Mulliken soon succeeded in assigning quantum numbers to electrons in
molecules. He presented his preliminary findings at the meeting of the
American Physical Society in February 1928,
(29)
and circulated the draft of
the paper, certain that his colleagues would find mistakes.
(30)
Mulliken first presented the aims of his program and then explained his
methods. He formulated a set of working rules for assigning quantum
numbers to electron states in molecules and gave examples of their application.
He cautiously noted that the method had so far been applied
exclusively to diatomic molecules made of atoms in the first row of the
periodic table. Only a few of the molecular states discussed were stable.
With an eye to future chemical applications Mulliken remarked that, besides
their purely theoretical importance, a knowledge of the numerous excited
states and chemically unstable molecules was indispensable in deducing the
electron configurations for stable molecules and the intermediate steps in
chemical reactions. The "essential ideas and methods" came from Hund;
Mulliken's achievement lay "in the attempt to assign individual electronic
quantum numbers" and obtain a knowledge of the energies of individual
electrons in molecules, similar to the existing knowledge of atoms.
(31)
In a
way, Mulliken attempted to assign electronic configurations to experimentally
observed molecular orbitals.
Hund had conceived of an adiabatic transition from the separated atoms
to the diatomic molecule and then to the united atom, thus supporting
Mulliken's earlier hypothesis that electronic quantum numbers could change
drastically in the process of molecule formation.
(32)
Mulliken thought more
along the lines of the old quantum theory rather than the new quantum
mechanics. He used neither Schrödinger's equation nor the language of
quantum mechanics. Mulliken's highly visual spectroscopic approach
seemed to be consistent with the existence of orbits. The only paragraph
where Mulliken addressed "the meaning of quantum states of electrons in
the new mechanics" was merely a cosmetic appendage to his largely "pre-
quantum mechanical" language.
By analogy to what Bohr had done in his "grand synthesis," Mulliken
pictured the molecules as being formed by feeding electrons into orbits
which encircled the nuclei.
(33)
As he recalled: "Bohr's
Aufbauprinzip for
atoms made a very great impression on me and so I thought something
similar for molecules would be nice. If you translate orbits into orbitals for
atoms, then for molecules it is molecular orbitals; it is something that goes
around all the atoms or however many atoms there are and the
Aufbauprinzip transferred to molecules simply means molecular orbitals."
(34)
To apply the Aufbauprinzip to molecules, two sorts of questions had to
be clarified. The first concerned the quantum numbers for electrons in
molecules and the nature of closed shells, molecular states, and multiplets.
The second concerned binding energies in the molecule and the energy relations
resulting from the union of the two atoms. To reply to the first set of
questions, theorists emphasized the relation between a molecule and a
molecule-as-united-atoms. To reply to the second, they emphasized the
relation between a molecule and the separated atoms.
Mulliken suggested that
the possible quantum numbers for each electron could be obtained from
those of the associated united atoms by placing the atoms in a strong axially
symmetric electric field, to fix the two nuclei in the molecule. He
justified this simplification on the ground that "we are not directly
interested here in the effects of nuclear rotation and vibration." Several
coupling schemes could be applied and, contrary to the atomic case, "in
molecules, no one limiting case is ordinarily approached, ..... . the actual
condition usually lies more or less in the midst of a region between several
limiting cases."
(35)
The relation to the separated atoms enabled Mulliken to discuss the
energy conditions favorable to the formation of the molecule. In order to
obey the Pauli principle for a molecule-as-united-atoms, some electrons
could have their value of n increased in the process of molecule formation.
Mulliken dubbed these "promoted electrons" and the associated increase in
energy, "energy of promotion." To analyze the energy necessary for the
formation of the molecule, Mulliken divided the total energy into two components:
the positive potential energy of nuclear repulsion and the negative
binding energies of each electron in the field of the nuclei and the other
electrons. To form a molecule, the electronic binding energy must increase
more rapidly than the nuclear repulsion energy for r>r0
(where r0 is the
internuclear equilibrium distance) For r = r0,
the two types of energy
increased at the same rate and the total energy of the molecule attained a
minimum. For r<r0,
the nuclear repulsion energy had to increase faster
than the electronic binding energy. To form a reasonably stable molecule,
"the binding energy has to increase considerably faster than the nuclear
energy over a considerable range of r values, as r decreases towards
r0".
(36)
As the nuclear distance diminishes, the binding energy of the unpromoted
electrons should increase steadily, since the electrons are influenced
by two nuclei, reaching a maximum when the molecule forms. In the case
of promoted electrons the binding energy may increase or decrease, because
the increase in effective nuclear charge is at least partially offset by the
increase in energy associated with the increase of the quantum number
n.
This qualitative analysis threatened some of the most cherished concepts in
chemistry. Although, following Lewis, electrons were usually classified as
either "bonding" (the paired electrons that hold the molecule together) or
"non-bonding," Mulliken concluded that various degrees of "bonding
power" could be assigned for various orbit types. Electrons could have
positive bonding power if their presence in a molecule made the dissociation
energy large, or the equilibrium internuclear distance small.
(37)
The converse was also true. Two possible definitions of bonding power
resulted:
energy-bonding power or distance-bonding power,
arising either by the
application of the energy criterion or the distance criterion, and a set of
rules for the analysis of spectroscopic data and for the assignment of
quantum numbers to electron states of actual molecules.
(38)
As will appear, these
considerations were at the heart of Mulliken's criticism of the valence
theory of Heitler and London.
Mulliken completed this phase of his work in 1928, as he moved to the
University of Chicago as an Associate Professor in the Physics Department.
In recognition of his outstanding contributions at New York University,
where he had moved after Harvard, Mulliken was offered several jobs, all
of them related to research programs on molecular structure: to replace
Loomis as head of the Physics Department at NYU and continue developing
the program he helped to start; to accept an offer from R.W. Wood of Johns
Hopkins University and start a program there on the study of molecules; to
go to Harvard to help Kemble and Slater develop a molecular research
program; or to accept the offer of A.H. Compton and the Department of
Physics of the University of Chicago.
Mulliken opted for Chicago. Besides sentimental reasons - according to
Slater, "he liked everything about the great city, even its gangsters"
(39)
- the physicists at Chicago, especially Compton and the spectroscopist H.A. Gale
(head of the Physics Department), were the most persuasive. Chicago
already possessed a good spectroscopic laboratory, Eckart Hall (designed by
the spectroscopist G.S. Monk), and Gale had promised Mulliken a new
high-resolution grating. Their program could expand with the
transformation of the Ryerson Laboratory into a molecular research center.
Endowments would fund new apparatus and the university had always been
willing to hire research assistants and pay visiting professors. Hund,
Heisenberg, and Dirac were to spend the summer of 1929 in Chicago.
{underlined by this website}.
 click to enlarge click to enlarge |
Slater was being pressed to join the department. Mulliken wanted to
develop a molecular program along theoretical as well as experimental
lines. He did not consider himself a theoretical physicist, but a sort of
middleman between experiment and theory; interaction with theoretical
physicists was crucial for "stimulus and cooperation." Mulliken was asked to
give an advanced undergraduate course and a graduate course that boiled
down to the supervision of three or four graduate students. Chicago offered
exactly what Mulliken was looking for: a minimum teaching load, just for
"stimulus," and expert colleagues in associated fields.
(40)
|
Due to a delay in getting the high-resolution spectrograph, Mulliken
shifted into more theoretical matters and wrote his review articles on "The
interpretation of band spectra," a series originally intended for a book.
(41)
The correlation diagram for homonuclear diatomic molecules appeared for
the first time in these articles. These diagrams provided a comparison of the
electronic energy levels of diatomic molecules (relative to the energy levels
of the separated atoms and corresponding united atom) as a function of the
internuclear separation. They completely described the electronic structure
of diatomic molecules made up of identical atoms. In their influential
review article of 1935, Van Vleck and Sherman recommended that the
correlation diagram be "placed on the walls of chemistry buildings, being
almost worthy to occupy a position beside the Mendeleeff periodic table so
frequently found thereon. Just as the latter affords an understanding of the
structure of atoms, so does the former afford an understanding of the structure
of molecules, with which the chemist is often concerned."
(42)
The review articles on the interpretation of band spectra and the notation
for diatomic molecules marked the end of Mulliken's work on the
spectra and structure of diatomic molecules.
(43)
He then shifted to the study
of polyatomic molecules and to problems related to valence. He also
wanted to disseminate his work on band spectra, his preliminary ideas on
the chemical bond, and his criticism of Heitler and London's suggestions.
In the meantime, another promising young American physical chemist
started to address the question of the chemical bond.
The young Pauling
Linus Pauling attended the California Institute of Technology from 1922
to 1925, studying for his Ph.D. He worked under R.G. Dickinson on the
determination of the structure of crystals by means of X-ray diffraction. He
stayed on at Pasadena one more year as a National Research Council postdoctoral
fellow. Pauling made his first contributions to the subject of the
chemical bond in this period.
(44)
 L. Pauling
L. Pauling
1901-1994
Photo from C.J. Ballhausen:
Quantum Mechanics and Chemical Bonding
in Inorganic Complexes,
J. Chem. Ed. 56, II: 294-297 (1979).
|
|
Although these contributions came within the framework of the old
quantum theory, they also contained the interplay of theory and experiment
that characterized Pauling's successful explanation of the chemical bond. In
one paper, Pauling used information on crystal structure together with
Lewis's shared-electron bond to suggest a new way to analyze the relative
stability of groups of molecules composed of the same atoms and having
the same total number of electrons.
(45)
In another, he represented Lewis's
shared electrons by means of binuclear orbits and, together with experimental
evidence from his work on crystal structure, suggested dynamic models
for the ammonium ion (NH4 +), benzene
(C6H6), and other aromatic
molecules.
(46)
By the end of 1925, the two papers on the chemical bond bad been sent
for publication. Following the advice of his mentor A.A. Noyes, Pauling
applied for a Guggenheim Foundation fellowship. He planned to stay in
Munich at Sommerfeld's Institute for Theoretical Physics for a year and to
visit Bohr in Copenhagen. He also planned to pay brief visits to other
centers where work on crystal structure, either theoretical or experimental,
was being carried out, including Born's Institute in Göttingen and the
Braggs's laboratory in Manchester. Pauling also arranged for a stay with
Schrödinger in Zürich.
|
Pauling adapted smoothly to Munich's scientific lifestyle. He attended
Sommerfeld's lectures on the new wave mechanics, which then had just
been formulated by Schrödinger. He realized how much he missed the
"invigorating discussions" of seminars devoted to "formal presentations"
of new papers, and not to presentations of students' research problems.
Pauling also realized that, in his first meeting with Sommerfeld, he had
undiplomatically suggested a research topic he would like to work on,
instead of waiting for Sommerfeld's advice. However, he feIt that he would
probably be assigned a problem that met his interest since electronic
motions in binuclear orbits were a topic of research at the Institute.
(47)
|
Sommerfeld suggested that Pauling work on the spinning electron,
(48)
but
Pauling mentioned his earlier paper on the effect of electric and magnetic
fields on the dielectric constant of hydrogen chloride, and Sommerfeld
allowed him to extend that work to fields of arbitrary strength.
(49)
Pauling compared the results obtained with the old quantum theory to those
obtained with the new mechanics, and proved that the new gave values of
the dielectric constants in good agreement with experiment. This result,
more than anything else, convinced Pauling that quantum mechanics was
necessary for the solution of chemical problems. He wrote to Noyes: "I
am now working on the new quantum mechanics, for I think that atomic
and molecular chemistry will require it. I am hoping to learn something
regarding the distribution of electron-orbits in atoms and molecules."
(50)
While Pauling was in Munich a new line of research emerged as a result
of a paper published by Gregor Wentzel, a former doctoral student of
Sommerfeld and a Privatdozent at the Institute. Taking into account the
electron spin and the new quantum mechanics, Wentzel calculated the screening
constants for electrons in complex atoms, but there was still poor agreement
with experimental results.
|
|

A. Sommerfeld, 1928
Photo from M. Eckert, Die Atomphysiker, Vieweg, Braunschweig, 1993.
|
Sommerfeld had introduced the concept of
screening of the outer electrons by inner ones to explain the fine-structure
of X-ray levels. Pauling examined this question systematically. He noticed
that some of the previous approximations were not correct and, when
properly refined, gave good agreement with experiment.
(51)
Although Noyes
advised Pauling to keep publishing in American journals while in Europe,
this paper, being a reply to Wentzel, came out in the Zeitschrift für
Physik.
(52)
Buoyed by this success,
Pauling continued to work on screening constants,
using quantum mechanics to study the electronic structure and physical
properties of complex atoms and ions.
(53)
Encouraged by Sommerfeld,
Pauling published his results in the Proceedings of the Royal Society of
London.
(54)
Pauling called this paper the "start of quantum mechanics of
polyelectronic atoms."
(55)
The Heitler-London paper of 1927
Look at their paper of 1927 :
1.) 1 chunk, 2.3MB, pure HTML, right
here!
or
2.)
page by page, 130 KB each, javascript needed,
at this place.

W. Heitler 1904-1981
Photo from here
: Theor.Physik,Univ.Frankfurt,Germany
|
In April of 1927, Walter Heitler and Fritz London, both on Rockefeller
fellowships, went to Zürich hoping to work with Schrödinger. Heitler had
initially intended to work with Nils Bjerrum in Copenhagen on problems of
solutions. He convinced the authorities to allow him to go to Zürich, since
he wanted to work in quantum mechanics. London had already been working
in quantum mechanics while assisting Ewald at Stuttgart, contributing
to what came to be known as transformation theory. He felt that
Heisenberg's matrix mecbanics, would be a useful guide for his research.
What they expected did not come to pass, since Schrödinger did not like
collaborations.
|

F. London 1900-1954
Photo from here
|
About a month after arriving at Zürich, Heitler and London decided to
calculate the van der Waals forces between two hydrogen atoms as "just a
small 'by the way' problem." Nothing indicates that Schrödinger gave
them the problem of the hydrogen molecule or that they talked with him
about it. Schrödinger knew about their work, however, and told Mulliken
that there were two people working in his Institute who had some results
"which he thought would interest me very much; he then introduced me to
Heitler and London whose paper on the chemical bond in hydrogen was
published not long after."
(56)
Heitler and London initially calculated the interaction of the charges of
two atoms "without even thinking of the exchange." They found that the
"Coulomb integral" could not account for the van der Waals forces: "So
we were really stuck and we were stuck for quite a while; we did not know
what it meant and did not know what to do with it." Heisenberg's work on
quantum mechanical resonance did not help them, since the exchange was
part of the resonance of two electrons, one in the ground state, the other
excited, and both in the same atom. Then came some hot air:
(57)
Then one day was a very disagreeable day in Zürich;
[there was the] Föhn.
It's a very hot south wind, and it takes people different ways. Some are very
cross... and some people just fall asleep...
I had slept till very late in the
morning, found I couldn't do any work at all... went to sleep again in the
afternoon. When I woke up at five o'clock I had clearly - I still remember it
as if it were yesterday - the picture before me of the two wave functions of
two hydrogen molecules joined together with a plus and minus and with the
exchange in it. So I was very excited, and I got up and thought it out. As
soon as I was clear that the exchange did play a role, I called London up; and
he came as quickly as possible. Meanwhile I had already started developing
a sort of perturbation theory. We worked together until rather late at night,
and by that time most of the paper was clear... Well, I am not quite sure if
we knew it in the same evening, but at least it was not later than the
following day that we knew we had the formation of the hydrogen molecule
in our
hands. And we also knew that there was a second mode of interaction which
meant repulsion between two hydrogen atoms - also new at the time - new to
the chemists too. Well the rest was then rather quick work and very easy,
except, of course, that we had to struggle with the proper formulation of the
Pauli principle, which was not at that time available, and also the connection
with spin... There was a great deal of discussion about the Pauli principle
and how it could be interpreted.
Heitler and London started by considering the two hydrogen atoms
slowly approaching each other. They assumed electron 1 to belong to atom
a and electron 2 to atom b, or vice versa. Because the electrons were
identical, the total wave function of the system was the linear combination
of the wave functions for both cases:
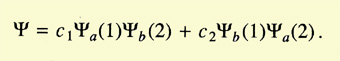 The problem was to calculate the coefficients c1 and c2.
They minimized the energy,
The problem was to calculate the coefficients c1 and c2.
They minimized the energy,
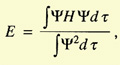
and found two values for it:
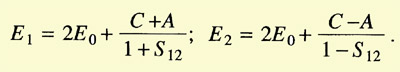
The integrals C (Coulomb integral) and A (exchange integral) had negative
values, A being larger than C. E1 implied
c1/c2 = 1
and E2 implied that
c1/c2 = -1. Hence the wave function of the system could now
be written as
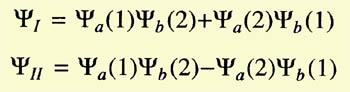
 |
|
They had not yet taken the spin of the electrons into account. The
symmetry required by the exclusion principle was satisfied only by
Ψ1, when
the electrons had antiparallel spins; but Ψ1
corresponded to E1. E1 was less
than 2E0, the sum of the energies of the two
separate hydrogen atoms, and
hence, it signified attraction. Ψ II,
which denoted parallel electron spins,
corresponded to E2. But E2 was greater than 2E0,
implying repulsion.
Bonding between two neutral hydrogen atoms could take place only when
the spins of the electrons were antiparallel. (This, according to Heitler and
London, was the justification for the electron pairing that Kossel - but not
Lewis - had talked about). An electron pair required not only energetically
available electrons, but also properly oriented ones. The homopolar bonding
could be understood as a pure quantum effect, since its explanation
depended wholly on the electron spin, which had no classical analog.
Heitler and London found the energy to be 72.3 kcals and the internuclear
distance 0.86 Angstroms (compared with the experimental values of 109.4
and 0.74).
(58)
|
Reactions were generally positive. London later told W.M. Fairbank
that Schrödinger had been very favorably surprised since he did not expect
his equation to describe the whole of chemistry.
(59)
Born and Franck also
were very enthusiastic about the paper. Sommerfeld initially responded
coolly, but he warmed up once Heitler explained certain points directly to
him.
The exchange force remained a mystery. Heitler and London had not
expected to find one, because they began with van der Waals forces in
mind.
(60)
The proposed exchange mechanism confronted them with a
fundamentally new phenomenon. Experimental physicists and chemists posed
questions about what was exchanged and the frequency of exchange:
(61)
I believe that for a long time I thought it was a major ununderstood problem
of quantum mechanics to explain what this exchange really means. We were
pressed by the questions of other people and one always likes to understand
the unfamiliar lines on the basis of something that is familiar with one. But
this was something quite new ... it became gradually clear to me that it
has to
be taken as a fundamentally new phenomenon that has no proper analogy in
older physics. And that is, I think, the only proper way to describe it. One
can define a frequency of exchange in a certain manner - one has to be very
careful about it - by a frequency of exchange of spin directions;
that is a possibility.
But this does not occur in the finished molecule; that would be a
non-stationary state where the spin directions exchange at the frequency that
is identical to the value of the exchange integral. There are many
misunderstandings
and I dare say that even today not all chemists, and not even all
physicists arc really clear about it. But I think the only honest answer today
is that the exchange is something typical for quantum mechanics, and should
not be interpreted - or one should not try to interpret it - in terms of
classical physics.
Both London and Heitler in all their early writings repeatedly stressed the
"non-visualizability" of the exchange energy; commentators have
consistently misinterpreted this.
Although the treatment of the homopolar bond of the hydrogen molecule
appeared to be an "extension" of the methods successfully used for the
hydrogen molecule ion, a radical difference existed between the two cases:
the role of the Pauli principle. John L. Heilbron, in his penetrating study of
the origins of the exclusion principle, called it "one of the oddest of the
instruments of microphysics," and stated that Pauli's first enunciation in
December 1924 had the form not of a dynamical principle but of the Ten
Commandments.
(62)
Hermann Weyl talked of Pauli's principle as something
which revealed a "general mysterious property of the electron."
(63)
Pauli's paper on spin appeared while London and Heitler were in
Zürich.
(64)
They judged it to be an unsatisfactory
"hybrid between a wave
equation and some matrix mechanics superposed on it. It was, so to speak,
glued together, but not naturally combined together."
(65)
Now the problem of
the hydrogen molecule ion could be solved by application of the
Schrödinger equation where only electromagnetic forces determine the
potential. A similar approach to the hydrogen molecule led to a physically
meaningless solution that did not account for the attractive forces. An
additional constraint was required. Heitler and London applied this constraint
not in the form of further assumptions about the forces involved, but as an
invocation of the Pauli exclusion principle, leading to a quite amazing
metamorphosis of the physical content of the mathematical solutions. The
terms previously giving strongly repulsive forces gave strongly attractive
forces under the new constraint. These terms not only became physically
meaningful, but their interpretation in terms of the Pauli principle led to a
realization of the new possibilities provided by the electromagnetic
interaction.
London proceeded to a formulation of the Pauli principle for cases with
more than two electrons, which proved convenient for his later work in
group theory: the wave function can contain arguments symmetric in pairs,
and electron pairs on which the wave function depends symmetrically have
antiparallel spin. He considered spin to be the constitutive characteristic of
quantum chemistry. Since two electrons with antiparallel spin are not
identical, the Pauli principle did not apply to them. The symmetric solution
therefore could be chosen:
(66)
The possibility of a homopolar chemistry rests quite essentially on the fact
that the electrons can still differ from each other in one respect, which we
have completely ignored until now: namely in the orientation of the rotation
axis of their eigen-rotational movement. There are so to speak two different
kinds of electrons, depending on the direction of their spin vectors (parallel
or antiparallel to a magnetically preferred direction),
and as the Pauli principle is only a statement concerning absolutely
identical particles, it implies no
restriction at all when the spin vectors point in opposite directions.
With the Pauli principle it became possible to comprehend "valence"
saturation: whenever two electrons of different atoms combine to form a
symmetric Schrödinger vibration, a bond will result. Spin became one of
the most significant indicators of valence behavior. As Van Vleck put it,
spin lay "at the heart of chemistry."
(67)
Polyelectronic molecules and group theory
By September 1927 Heitler had gone to Göttingen as Born's assistant
and London to Berlin as assistant to Schrödinger (who had succeeded
Planck). Heitler found physics at Göttingen exciting, especially Born's
course in quantum mechanics, which started with the matrix forinulation
and then derived "God knows how' Schrödinger's equation."
(68)
Heitler felt
that group theory provided the only solution to the many-body problem, and
he outlined his program to London in two long letters.
Heitler began by clarifying the meaning of the line chemists drew
between two atoms in diagrams. He held that every bond line represented
the exchange of two electrons of opposite spin between two atoms. He
examined the case of the nitrogen molecule and, in analogy with hydrogen,
the term containing the outermost three electrons of each atom. Their spins
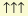 and
and
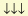 )
signified attraction:
(69)
)
signified attraction:
(69)
The general proof for something like this cannot be given, except group
theoretically. In all probability it is about the theory of reducible
representations, which as representations of the totality of permutations,
e.g. of 6 elements, is not possible to be further reduced. But now it
should be considered
as a representation of a subgroup with 3 elements, and as such they are not at
all impossible to reduce. It appears to be quite difficult and in any case
it is
necessary to dip into group theory.
Let us assume for the moment that the two atomic systems
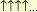 and
and
 are always attracted in a homopolar manner. We can, then, eat
Chemistry with a spoon.
are always attracted in a homopolar manner. We can, then, eat
Chemistry with a spoon.
Heitler's program to explain all of chemistry got him into trouble more
than once. Wigner used to tease him. He would ask: " ' what chemical
compounds would you predict between nitrogen and hydrogen?' And of course,
since he did not know any chemistry he couldn't tell me."
(70)
Heitler
confessed as much: "The general program was to continue on the lines of the
joint paper with London, and the problem was to understand chemistry.
This is perhaps a bit too much to ask, but it was to understand what the
chemists mean when they say an atom has a valence of two or three or
four ... Both London and I believed that all this must be now within the
reach of quantum mechanics."
(71)
Heitler then worked out in detail the methane molecule CH4. C is
in the
state  (C has to be excited from its ground state in order to be in
(C has to be excited from its ground state in order to be in
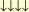 which agrees with experience). Four different "cells" in the L-
shell exist for four electrons combined antisymmetrically. The four
hydrogen atoms accordingly would be
which agrees with experience). Four different "cells" in the L-
shell exist for four electrons combined antisymmetrically. The four
hydrogen atoms accordingly would be
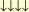 .
Methane could therefore be
reduced to a simple formula: the four hydrogen atoms are attracted in a
homopolar manner to the carbon atom, without, however, any repulsion
among them. The tetrahedron resulted. It was all very promising: "The long
story for the foundational aspects of this matter, could give us, I believe, a
series of very simple and equally interesting observations, if it were
possible to approximate better the whole damn thing."
(72)
.
Methane could therefore be
reduced to a simple formula: the four hydrogen atoms are attracted in a
homopolar manner to the carbon atom, without, however, any repulsion
among them. The tetrahedron resulted. It was all very promising: "The long
story for the foundational aspects of this matter, could give us, I believe, a
series of very simple and equally interesting observations, if it were
possible to approximate better the whole damn thing."
(72)
London agreed with Heitler that group theory could help generalize the
results derived by perturbation methods. They hoped to prove that, among
all the possible combinations of spins between atoms, only one term
provides the necessary attraction for molecule formation. Nevertheless, unlike
Heitler in the company of Wigner and Weyl at Göttingen, London was not
carried away by the spell of the new techniques. London "did not join in
[Heitler's] studies of group theory. He thought it was too complicated and
wanted to get on in his own more intuitive way."
(73)
Wigner's papers implied that group theory could be used for classifying
the energy values in a many-body problem as well as for calculating
perturbation energies. The theory of the irreducible representations of the
permutation group provided the possibility of dealing mathematically with the
problems of chemical valence and avoided the difficulties involved in
many-body problems. This lack of reliable methods for tackling many-
body problems haunted London all his hife. Many years later, however, this
difficulty helped him articulate the concepts related to macroscopic quantum
phenomena.
Not everyone welcomed the use of group theory in physics. Hartree
wrote to London:
(74)
I am afraid that having studied physics, not mathematics, I find group theory
very unfamiliar, and do not feel I understand properly what people are doing
when they use it. (In England, "Physics" usually means "Experimental
Physics," until the last few years "Theoretical Physics" has hardly been
recognized like it is here [at the time Hartree was in Copenhagen], and, I
understand, in your country. In Cambridge particularly the bias has been very
much towards the experimental side, and most people now doing research in
theoretical physics studied mathematics, not physics). I have been waiting to
see if the applications of group theory are going to remain of importance, or
whether they will be superseded, before trying to learn some of the theory, as
I do not want to find it is going to be of no value as soon as I begin to
understand something about it! 15 it real!y going to be necessary for the
physicist
and chemist of the future to know group theory? I am beginning to think it
may be.
After moving to Göttingen in late 1927, Heitler published papers
dealing with valence by group theory. In a significant paper with Rumer, he
studied the valence structures of polyatomic molecules and determined the
closest possible analog in quantum mechanics to the chemical formula
which represented the molecule simply by fixed bonds between two adjoining
atoms.
(75)
They found that the bonds were not strictly localized.
Nevertheless, in general the dominant structure corresponded to the chemical
formula. But other structures turned up that also were useful in understanding
chemical reactions:
(76)
And it was really in this paper that we then could see what the chemists mean
by a chemical formula, when they write down a chemical formula .... There
were a few new things for the chemists; one is that to each chemical formula
there corresponds a wave function, but the wave function is not such that it
corresponds to one chemical formula alone, but is in general a combination of
several. There are some structures which are quite important for the
calculation of energy contribution which the chemists never think of.
London was
the first [a long time before the Heitler-Rumer paper] who showed that the
activation energies in the treatment of the three hydrogen atoms could be
understood in quantum mechanics, and this method gave us then a general
understanding for it. Later Pauling called this a resonance between several
structures, which is a name perhaps not quite in agreement with the use of the
word resonance by physicists ... A further point which was violently objected
to by the chemists was that both London and I stated that the carbon atom
with its 4 valences must be in an excited state ... all this was later
accepted by
the chemists, but at that time I don't think the chemists did find this
of much use for them.
Convinced that it was impossible to continue his work in chemical
valence by more analytic methods, London also turned to group theory, for
three reasons. Firstly, the correlation between qualitative assessments of a
theoretical calculation and the "known chemical facts" confirmed the
methodology chosen by expressing the observed regularities as rules.
Secondly, since analytic calculations were hopelessly complicated, group
theory was the only convenient way to deal with the valence numbers of
polyelectronic atoms. Thirdly, the interpretation of the chemical facts was
compatible with the conceptual framework of quantum mechanics. Using
group theory it might be possible "to discover in the quantum mechanical
description conceptual facts which in chemistry have proven themselves in
complicated cases as a guide through the diversity of possible combinations,
and see them in their connection with the structure of atoms."
(77)
London
attempted to interpret the valence numbers of the homopolar combinations
based "on the conceptual representations" of wave mechanics, similar to
the interpretation of the polar valence numbers by Kossel and Lewis.
By the middle of 1928, London had drawn up a three-pronged program
to tackle "the most urgent and attractive problem of atomic theory: the
mysterious order of clear lawfulness, which is the basis for the immense
factual knowledge of chemistry and which has been expressed symbolically
in the language of chemical formulas."
(78) He intended to study the mutual
force interactions between the atoms; he wished to examine the
semi-empirical rules that chemists had found and to place them on a sound
theoretical basis; and he would attempt to determine the limits of these
rules and, if possible, treat them quantitatively.
But he doubted that the principles considered so far in atomic theory
would enable him to realize such a program. The characteristic interaction
of the chemical forces deviated completely from other familiar forces:
chemical forces seemed to "awaken" after a previous "activation," and
they suddenly vanished after the "exhaustion" of the available "valences."
Elementary symmetry considerations showed that the homopolar valence
forces could be mapped onto the symmetry properties of the Schrödinger
eigenfunction of the atoms and could be interpreted as quantum-mechanical
resonance effects. This interpretation was formally equivalent to its chemical
model; it produced the same valence numbers and it satisfied the same
formal combination rules, as expressed in the symbolic representation of the
structural formulas of chemistry. These followed from group theory as an
immediate consequence of the Pauli principle; the valences "saturated"
because the Pauli principle restricted the occupation of equivalent states.
Group theory demonstrated that the "uniqueness of the chemical symbolism
is actually a consequence of the most fundamental theorems of the theory
of the representations of the symmetric group."
(79)
London's "spin theory of valence" dealt mainly with cases in which
each electron in a pair comes from a different atom. He examined the
condition that electrons from different atoms can combine with each other into
a spin-zero pair. An electron already paired with another electron in the
same atom did not come into consideration. But a paired electron could
become available for bonding with an electron from another atom if it could
be unpaired without the expenditure of much energy. London claimed that
an electron could be unpaired provided that its total quantum number n did
not change. He considered the unpairing an intennediate step in the
formation of a compound.
(80)
Reactions to the Heitler-London paper
The Heitler-London paper opened a new era in chemistry. Quantum
mechanics led to the significant conclusion that two hydrogen atoms form a
molecule and two helium atoms do not. Such a "distinctjon is characteristically
chemical and its clarification marks the genesis of the science of
subatomic theoretical chemistry," remarked Pauling.
(81)
Van Vleck wrote:
"Is it too optimistic to hazard the opinion that this is perhaps the begin-
nings of a science of ' mathematical chemistry ' in which chemical heats of
reaction are calculated by quantum mechanics just as are the spectroscopic
frequencies of the physicist?"
(82)
Both de Broglie and von Laue regarded the paper as a classic.
(83)
In their
book on quantum mechanics for chemists, Pauling and E. Bright Wilson
hailed Heitler and London's work as the "greatest single contribution to the
clar;fication of the chemists' conception of valence" since Lewis's suggestion
of the electron pair.
(84)
Heisenberg considered the theory of valence of
Heitler and London to "have the great advantage of leading exactly to the
concept of valence which is used by the chemist."
(85)
Buckingham quoted
McCrea, who recalled his own attempts to solve the problem of the hydrogen
molecule bond:
(86)
This was the most important problem I considered these days, but I got
nowhere. Then one day in 1927, I was able to tell Fowler that a paper by
Walter Heitler and Fritz London apparently solved the problem in terms of a
new concept: a quantum mechanicai exchange force. He grasped this idea at
once, and bade me to expound it in the next colloquium - which is how
quantum chemistry came to Britain.
Although "impressed with the skill" of London's papers, Wigner
thought that "the quantum mechanics of chemistry is not a resounding
success ... the Heitler-London paper is very beautiful, but much of what
came afterwards is only ' all right '."
(87)
London's papers on group theory
provoked positive comments by Weyl in his book Group theory and quantum
mechanics.
(88)
Born thought related the group theoretical treatment so
"difficult and involved" that few people could definitely state what it had
achieved, and stressed the importance of Weyl's generalization of it.
In 1928 two review articles appeared that exerted a deep influence on
chemists. Published in the Chemical Reviews, both had the explicit aim of
"educating" the chemists in the ways of the new mechanics.
(89)
Pauling
presented the details of Burrau's calculation of the electron charge density
distribution of the hydrogen molecule ion.
(90) Pauling considered the
Heitler-London treatment of the structure of the hydrogen molecule "most
satisfactory" and repeatedly stated that spin and resonance would explain
chemical valence. Concerning the recent work of Heitler, London, and
himself, Pauling commented that their results agreed with the qualitative
conclusions of Lewis:
(91)
When I examined the papers pubiished by Heitler and London, I was unable
to formulate a good relationship between their calculations and my
knowledge of the properties of atoms and molecules. I realized that the
chemists' ideas about the sharing of electron pairs fit more closely with the
theory of quantum mechanics if the assumption of Russell-Saunders coupling
were to be abandoned for atoms in molecules. In 1928 I published a short
paper in the U.S.A. Proceedings of the National Academy of Sciences, in
which I discussed briefly a number of results obtained in this way, including
the statement that the resonance phenomenon in quantum mechanics gives
rise to the tetrahedral arrangement of the four single bonds formed by a
quadrivalent carbon atom in the molecules. I mentioned that a detailed
account of the calculations wöuld be published iater. In fact, the
calculations
that I made were so complicated that I felt there was some uncertainty about
convincing other people of their correctness, and it was not until three years
later that I was able to formulate a remarkable method of simplifying the
quantum mechanical treatment of the chemical bonds and to publish a long
paper on application of quantum mechanics to the problem of the nature of
the chemical bond.

John Van Vleck
1899–1980
Photo taken from
J. Chem. Ed. 56, 296 (1979).
|
|
Van Vleck's review of quantum mechanics concentrated on explaining
the principles and the internal logic of the new theory.
(92) He gave
full credit
to the work of Heitler and London, something found in most of Van
Vleck's papers through 1935
(93) before going over to use
the more "practical" methods of Pauling and Mulliken.
He summarized the achievements
of quantum mechanics in physics in ten points and titled the section about
chemistry, "What the quantum mechanics promises to do for the chemist."
He emphasized the importance of spin for chemistry and showed that the
Pauli exclusion principle provided a remarkably coherent explanation of the
periodic table. He stressed its extreme importance elsewhere as well: "The
Pauli exclusion principle is the cornerstone of the entire science of
chemistry."
(94)
Van Vleck shared Dirac's attitude that the laws for the "whole of
chemistry are thus completely known" and thought that the dynamics that
successfully explained atomic energy levels for the physicist should support
calculations of molecular energy levels for the chemist.
|
The calculations,
though formidable, were unavoidable: the mathematical problem confronting
the chemist was "to investigate whether there are stable solutions of the
Schrödinger wave equation corresponding to the interaction between two
(or more) atoms, using only the wave functions which have the type of
symmetry compatible with Pauli's exclusion principle." Van Vleck drew
attention to the crucial feature of London and Heitler's approach, lest
chemists "get the wrong idea." The absence of certain compounds was not
because the calculations yielded energetically unstable combinations, but
because the corresponding solutions of the Schrödinger equation did not
satisfy the symmetry requirements of the Pauli principle: "Thus considerations
of symmetry (group theory) are often quite as vital as those of
energetics. The failure of the chemist to find a compound does not necessarily
mean that the corresponding molecule is ' energetically unstable, but may
mean rather that it would demand electronic groupings contrary to the
exclusion principle."
(95)
Few other papers at that time did so
much to advocate quantum mechanics for chemists.
Among the meetings that discussed chemical bonding, one in particular
expressed the radical changes occurring in the chemists' culture. The
chemists in the 1928 meeting of the American Chemical Society appeared
sufficiently fluent in the new physics, as appears from G.L. Clark's open-
ing remarks:
(96)
It may be asserted, in spite of discrepancies and disagreements, that in the
first quarter of the twentieth century, the actual existence of an atomic
world
became an established fact. Part of the difficulties in details may be
ascribed
to failure of chemists to test their well-founded conceptions with the facts
of
physical experimentation, and far too few physicists inquired critically into
the facts of chemical combination. So, firmly entrenched each in his own
domain, a certain long-range firing of static cubical atoms against
infinitesimal solar atoms has ensued, with few casualties and few peace
conferences. The position of the Bohr conception has seemed so convincing
that perhaps the majority of thinking chemists were coming to accept the
dynamic atom, which is fully capable of visualization.
Clark was not alone in specifying the problematic relationship between
physicists and chemists. Worth Rodebush, one of the first to receive a
doctorate in 1917 from the newly established Department of Chemistry at
Berkeley under the chairmanship of Lewis, went a step further than Clark.
The divergent paths of physicists and chemists were drawn together after
the advent of quantum theory and especiaily after Bohr's original papers.
But in this process, "the physicist seems to have yielded more ground than
the chemist. The physicist appears to have learned more from the chemist
than the chemist from the physicist. The physicist now tells the chemist
that his way of looking at things are really quite right because the new
theories of the atom justify that interpretation, but, of course, the chemist
has known all the time that his theories had at least the justification of
correspondence with a great number and variety of experimental facts." He
gracefully remarked that the physicist enabled him to calculate the energy
of formation of the hydrogen molecule by using the Schrödinger equation.
But the difficulty in a theory of valence was not to account for the forces
that bind the atoms into molecules; the outstanding tasks were to predict the
existence and absence of various compounds, and the unitary nature of
valence as expressed by the law of multiple proportions. The "brilliant
theories" of Lewis accounted for the features of valence "in a remarkably
satisfactory manner, at least from the chemist's point of view."
(97)
Rodebush
considered London's group theoretical treatment of valence to be important
even though it did not answer all the queries of the chemist.
Perhaps the most cogent manifestation of the characteristic approach of
American chemists was Harry Fry's articulation of what he called the
pragmatic outlook. He posed a single question for organic chemists: what
modifications to the structural formulas would conform with the current
concepts of electronic valence? No change should obscure the fundamental
purpose of a structural formula, which was to present the number, the kind,
and the arrangement of atoms in a molecule as weh as to correlate the
manifold chemical reactions displayed by the molecule:
(98)
It should here be noted that no theory in any science has been so marvelously
fruitful as the structure theory of organic chemistry.... When we are
considering methods of modifying this structure theory of organic chemistry,
by
imposing upon its structural formulas an electronic valence symbolism, are
we not, as practical chemists, obligated to see to it that such a system be
one
that is calculated to elucidate our formulas rather than render them obscure
through the application of metaphysically involved implications on atomic
structure which are extraneous to the real chemical significance of the
structural formulas, per se .... the opinion is now growing that the
structural formula of the organic chemist is not the canvas on which the
cubist artist
should impose his drawings which he alone can interpret .... Many chemists
believe that the employment of a simple plus and minus polar valence
notation is all that is necessary, at the present stage of our knowledge,
to effect
the further elucidation of structural formulas.
On the grounds that practical
results are the sole test of truth, such a simple system of electronic valence
notation may be termed "pragmatic."
"Chemical pragmatism" resisted the attempts to embody ' in the structural
formulas what Fry considered to be metaphysical hypotheses: questions
related to the constitution of the atom and the disposition of the
valence electrons. The chemical behavior of molecules was the primary
concern of the pragmatic chemist, rather than the imposition of an electronic
notation complicated by metaphysical speculations. Fry had to admit
that as chemists learned more about the constitution of the atom, they
would be able to exphain chemical properties more fully.
At the meeting of the Faraday Society in 1929, London (and to a lesser
extent Heitler) appeared to agree with the molecular orbital approach as a
way of providing a quantitative dimension to the valence group theory. This
was not the sole reason for the formulation of the molecular orbital
approach, an obvious point needing emphasis nonetheless since the widely
held view that regards the molecular orbital approach as diametrically
opposed to the valence bond method, while methodologically justified,
appears to be historically untenable. Hund addressed a weakness of the
group theoretical approach: chemical binding could not be understood in
terms of energetics; the saturation of the valences was explainabhe only in
terms of spin,
(99)
and he suggested mechanisms for molecule formation to
account for the characteristic differences between H2 and HeH.
Lennard-
Jones proposed a set of rules for the assignment of electrons to molecules
consistent with the group theoretical considerations of both London and
Heitler - especially with London's suggestion of two kinds of unsaturated
valences that could give rise to molecular binding: one owing to an incomplete
group of electron spins, the other to an incomplete group of angular
momentum spins.
(100)
Pauling introduces resonance
After staying with Sommerfeld in Munich for approximately thirteen
months, Pauling went to Copenhagen, where he stayed less than a month.
There he worked with Goudsmidt on the hyperfine structure of bismuth
without much success. He then moved to Zürich, where he stayed for about
two months. On the whole, he "rather regretted" the time spent in Zürich.
He saw Schrödinger only at the weekly seminars, never found out what he
was working on, and did not manage to interest him in his own work. "I
offered to make any calculation interesting to him since he was not
interested in my work; but without success."
(101)
He met Heitler and
London, who, shortly before his arrival, had finished writing their paper on the
formation of the hydrogen molecule, but they did not invite his collaboration.
Thus, he continued alone to muse over the nature of the chemical
bond.
Pauling's notebooks display some of his early thoughts about the nature
of the chemical bond.
(102)
The first set of notes are from 1926, when he was
still in Munich. Before his trip to Europe, Pauling had thought about the
electron-pair bond in terms of electrons in binuclear orbits,
(103)
and in his
application for the Guggenheim Fellowship he proposed to apply quantum
theory to their study. Burrau was the first successfully to integrate the wave
equation for the simplest molecule - the hydrogen-molecule ion.
(104)
He found
a numerical expression for the electronic wave function in the field of the
two nuclei - that is, he obtained the first numerical expression of a moledular
(binuclear) orbital, together with values for the equilibrium internuclear
distance, total energy, and vibrational energy of the lowest state.
In order to study diatomic molecules of identical atoms, Pauling tried to
determine the form of molecular (binuclear) orbitals by approximating the
internuclear potential by two identical one-dimensional square-well potentials.
He then integrated Schrödinger's equation for the electron in the
different regions of this simplified potential. He used the boundary conditions
to determine some of the constants of integration and analyzed the
extreme situations corresponding to the separated atoms (infinite distance
between the atoms) and the united atom (zero distance between the atoms).
The eigenfunctions obtained were either symmetrical or antisymmetrical in
the position coordinate, implying symmetrical or antisymmetrical electron
orbits. Then, by assuming that shared electrons were necessarily symmetrical
in two-center orbitals (whereas unshared electrons were half symmetrical
and half antisymmetrical), Pauling wrote the Lewis formulas for
F2, 02 and N2. Where more than one formula
could be written, he used the
available empirical information together with his theoretical results to devise
rules to decide among them. He noted that "the spinning electron accounts
for electron pairs." He also referred to resonance energy: "There is, of
course, a continual interchange of energy among the various electrons, so
that one electron cannot be assigned to a given state; and there is the
corresponding resonance effect on the energy of the system."
(105)
Finally, he
tried to extend his square-well potential model to the case of a diatomic
molecule composed of two different atoms, and of polyatomic molecules
composed of two large and two small atoms.
Pauling was thinking about the electron-pair bond in terms of a Burrau-
like two-nuclei orbital, but he could not derive any results he considered
worth publishing. If the electrons had spin, then the Pauli principle implied
that they should have opposite spins. In his notes Pauling never stated this
consequence explicitly, but he later recalled having discussions with several
people "about the explanation of chemical bonding in terms of the Burrau
paper on the hydrogen molecule ion and the Pauli principle."
(106)
He might
have been thinking already of the electron pair bond in terms of two electrons
with opposite spins in a two-center molecular orbital.
While still in Munich, Pauling learned about Condon's "empirical
method" for the hydrogen molecule,
(107)
and was immediately struck by
"Condon's ingenuity." Condon assumed that the interaction of the two
electrons in the hydrogen molecule was small in comparison with the
electron-nuclei attraction, and represented each electron by
an H2 + eigenfunction.
To estimate the value for the perturbation energy, Condon
assumed the same relation between interelectronic energy and total energy
for the hydrogen molecule and for its united atom (the helium atom), and
used the known value for the latter to estimate the value for the former. In
this way Condon "got results as good as Heitler and London got later."
(108)
Although Pauling kept on thinking about the chemical bond while in
Copenhagen, his next set of important notes were written in Zürich.
(109)
There, Pauling discussed the chemical bond with Heitler and London. He
did not agree entirely with their results, an understandable reaction since for
more than three years he had been thinking about the electron pair bond as
a pair of electrons in molecular (binuclear) orbits.
(110)
In Munich he had
made the first calculations using a very rough approximation to the molecular
potential. Calculations with molecular orbitals were not easy. Burrau
and Condon used molecular orbitals for the hydrogen molecule ion and the
hydrogen molecule, respectively, but the integrations had not been
straightforward. In the case of the hydrogen molecule, Condon's ingenious
"empirical method" enabled him to circumvent some mathematical obstacles
and arrive at a good estimate for the interaction energy. Burrau and
Condon's success undoubtedly reassured Pauling about the potential for a
method alternative to that of Heitler and London. In Zürich he labored to
develop such an alternative computational method. In doing so he used both
the idea of resonance and the Pauli principle, which he later classified as
the two fundamental factors influencing chemical valence."
(111)
Pauling took two hydrogen atoms separated by a distance d and
represented each electron by an H2 + wave function,
as Condon had; according
to earlier calculations,
(112)
the function could be either
symmetric (Ψ) or
antisymmetric (Φ) relative to the nuclei. By also neglecting at first the
repulsion between the two electrons, Pauling approximated the wave function
for the hydrogen molecule by the product of the two electronic wave
functions; that is, Ψ(H2) = Ψ1Φ;2
(or Ψ2Φ;1).However, the indistinguishability
of the electrons produces a resonance or exchange phenomenon, so
that two different complete eigenfunctions can be formed for the hydrogen
molecule:
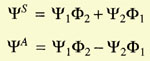 Taking the Coulomb interaction between the two electrons as the perturbation
force, represented by f = e2 /r12 , Pauling used perturbation theory to
express the perturbation energy due to the electrons:
Taking the Coulomb interaction between the two electrons as the perturbation
force, represented by f = e2 /r12 , Pauling used perturbation theory to
express the perturbation energy due to the electrons:
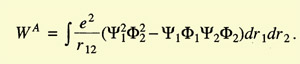 Representing the two-center wave functions in terms of one-center wave
functions Ψ and Φ
Representing the two-center wave functions in terms of one-center wave
functions Ψ and Φ
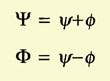 and substituting in the expression for the perturbation energy, Pauling
obtained
and substituting in the expression for the perturbation energy, Pauling
obtained
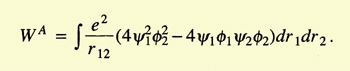 The first term represents the electrostatic repulsion between the electrons
and the second term the resonance between them.
The first term represents the electrostatic repulsion between the electrons
and the second term the resonance between them.
Pauling next considered the electrons in the two hydrogen atoms as
spinning particles. He distinguished six different cases: one with both electrons
represented by the symmetric H2 + eigenfunctions;
another with both
electrons represented by the antisymmetric H2 +
eigenfunctions; and four
with one symmetric and one antisymmetric H2 +
eigenfunction. Pauling concluded
that the latter four situations corresponded to the formation of the
hydrogen molecule from neutral atoms, whereas the other two corresponded
to the formation of the hydrogen molecule from the ions H+
and H-. By
representing the two-center wave functions (molecular orbitals) in terms of
one-center wave functions (atomic orbitals), Pauling could relate his six
cases (with two-center wave functions) with the four cases considered by
Heitler and London (with one-center wave functions). As we know, Heitler
and London obtained three cases where the electrons attracted each other
and one in which they repelled; they did not consider the formation of the
hydrogen molecule from two ions, so they had two solutions less than Paul-
ing. Pauling proved that by starting with two separated like nuclei (one-
center wave functions) and two electrons, and taking into account the
degenerations owing to similar nuclei and indistinguishable electrons, one
would arrive at the six cases he obtained with two-center wave functions.
He analyzed his conclusions in his notebooks:
(113)
London and Heitler have a different method of treatment, starting with
separate atoms (or ions). Their eigenfunctions are not those obtained by
starting
from H2 + eigenfunctions.
I suppose, though, that they are roughly correct
for large distances, and that in some way there is transition to the
H2 + eigenfunctions,
probably through degeneration.
Thus if it could be shown that their H+ + H-
and H + H eigenvalues approach each other; it would be satisfactory.
There would have to be triple degeneracy - both
H+ + H- and one H + H coming together.
I suppose that this is what happens, and that neither my treatment nor
London's and Heitler's is correct, but that the correct treatment of the
secular
problem would give London and Heitler's results for large distances, and
mine for small, with intermediate ones in between, and with degeneracy at
points, so that all the transitions indicated are possible adiabatically
(switchings being called adiabatic).
Thus both H+ + H- and
H + H could go adiabatically to H2.
Besides working on the interaction between two hydrogen atoms, Pauling
spent most of his time in Zürich on the interaction between two helium
atoms, but he ran into some integrals for which he could not find good
approximate values. But he persevered, convinced that "if I worked in this
field I probably would find something, make some discovery, and that the
probability was high enough to justify my working in the field. Of course,
it led to hybridization and all of this stuff."
(114)
1931: One valence theory
Pauling returned to the California Institute of Technology in the fall of
1927 as Assistant Professor of Theoretical Chemistry. By 1931 he was a
Professor and the first recipient of the A.C. Langmuir Prize of the American
Chemical Society. During that time, he avidly followed the papers on group
theory of Heitler and London; in contrast to a number of physicists he was
at ease with group theory, although it did not appeal very much to him."
(115)
Among his extensive notes on their papers, and in his comments on one of
London's papers, is the first mention of the equivalence between the spin
theories of valence and Lewis's empirical ideas of the electron-pair bond,
the first thoughts on "changed quantization," and the first reference to how
Pauling intended to clarify the nature of the chemical bond."
(116)
In his important paper, "The shared-electron chemical bond,"
(117)
Pauling pointed out that chemical valence was the result of two factors: the
Pauli exclusion principle and the quantum mechanical resonance
phenomenon of Heisenberg and Dirac. Pauling recalled that resonance had
been an essential ingredient in Heitler and London's explanation of the
formation of the H2 molecule and emphasized the relation to Lewis's
theory:
Heitler and London's theory often was "entirely equivalent" to Lewis's
phenomenological theory of the shared pair bond. The quantum mechanical
explanation specified the meaning of a shared electron bond - two electrons
belonging to different atoms, in identical states, and with opposite spins.
But Pauling already had realized that the quantum mechanical explanation
of valence provided a "more detailed" and "more powerful" picture than
the old description. If the extension of London's theory had already
produced results, more were still to come.
 John C. Slater
John C. Slater
1900-1976
Photo © American Institute
of Physics |
Pauling further predicted that quantum mechanics alone could not solve
the problems posed by valence theory. "It is to be especially emphasized
that problems relating to choice among alternative structures are usually not
solved directly by the application of the rules resulting from the mechanics;
nevertheless, the interpretation of valence in terms of quantities derived
from the consideration of simpler phenomena and susceptible to accurate
mathematical investigation by known methods now makes it possible to
attack them with a fair assurance of success in many cases."
(118)
He hinted
at what he later called hybridization (which he then called "changed
quantization") and promised a detailed account of his claims on "changed
quantization." It took him three years to deliver his promise. The paper,
which Pauling classified as his single most important contribution to the
understanding of the chemical bond,
(119) came out almost simultaneously
with one by the physicist John Slater containing many of the same suggestions.
At the same time, Mulliken was proposing an alternative approach to
the problem of the chemical bond.
|
In 1931, Pauling went to Berkeley to deliver a series of lectures on
"The nature of the chemical bond," which spanned almost a month, from
March 23 to April 20. Birge enthusiastically wrote to Mulliken:
(120)
Pauling has just finished a series of 12 lectures here, which I think are the
most exciting lectures I ever attended. The main subject matter of the
lectures is contained in his article in the April J.A.C.S. [ref.
(124) ]
I think this is
an article of the very highest importance, and I hope you will read it
carefully, if you have not already done so. He has gotten many results since
this
article was sent in for publication... He is interested in the actual
character
of the binding near equilibrium, and I think he is really on the right track.
He is of course duplicating some of Slater's work, but according to him, this
is his idea, and he told Slater about it, some time ago. (See his paper in
P.N.A.S. in 1928, I think [ref.
(111) ].)
Certainly we are on the threshold of a
very large new development.
Confident of the importance of the results that had eluded him for nearly
three years, Pauling wrote a letter to A.B. Lamb, the editor of the Journal
of the American Chemical Society, urging him to publish the paper as soon
as possible. In his letter to Lamb, Pauling wrote:
(121)
The paper could be shortened a little by omitting references to previous work
and by condensing the explanatory discussions of new results; but I feel that
this would render it more difficult for chemists to understand what has been
done.
The paper seems to me to be primarily of chemical interest, and I hope
that it may be published in the Journal of our American Chemical Society;
but if prompt publication might be prevented through time-consuming
consideration by referees,
I must ask you to return the manuscript for I shall then
feel obliged to publish it in the Physical Review or in an European journal
where quick publication can be secured.
Pauling also sent a letter to the Physical Review in which he called the
attention of physicists to the article in the Chemical Society's Journal,
outlined its main conclusions, and emphasized the differences between his
paper and an earlier note by Slater.
(122)
Pauling made evident his debt to Lewis. "[I considered myself] not a
stranger bringing something from outside, but rather a member of the group
here carrying on the work begun by Professor Lewis in 1916; for ever since
I first learned of the electron-pair bond, in 1920, I have devoted my efforts
to attempting to understand the properties of substances from this
viewpoint, so that even though I never matriculated in the University of
California, I like to consider myself as to some extent a student of
Professor Lewis's."
(123)
In the opening paragraph of the first paper in the series in the Journal
of the American Chemical Society, Pauling assessed the work on the
chemical bond and outlined his own method:
(124)
During the last four years the problem of the nature of the chemical bond has
been attacked by theoretical physicists, especially Heitler and London, by the
application of quantum mechanics. This work has led to an approximate
theoretical calculation of the energy of formation and of other properties of
simple molecules... and has also provided a formal justification of the rules
set up in 1916 by G.N. Lewis for his electron bond. In [this] paper it will be
shown that many more results of chemical significance can be obtained from
the quantum mechanical equations, permitting the formulation of an extensive
and powerful set of rules for the electron-pair bond supplementing those of
Lewis. These rules provide information regarding the relative strengths of
bonds formed by different atoms, the angles between bonds, free rotation or
lack of free rotation about bond axes, the relation between the quantum
numbers of bonding electrons and the number and spatial arrangement of the
bonds.
Pauling considered his own work as an extension of the program of the
theoretical physicists who had applied quantum mechanics to chemistry.
He provided "many more" results and rules, which he sketched out using
approximations and arbitrary assumptions which might have inhibited a
theoretical physicist. He postponed the quantum mechanical justification of
the rules to the end of the lectures, but never gave them because of lack of
time. Their justification was not important; their usefulness in describing
the different bonds in chemical substances alone mattered.
(125)
Pauling implicitly assumed that the presence of other atoms in the
molecule did not much influence the conditions for bond formation. He
stated that bonds resulted from the overlapping of two atomic eigenfunctions,
the larger the overlap the stronger the bond. The bond formed in the
direction of the highest concentration of individual bond eigenfunctions.
The strength and directional character of bonds was thus a consequence of
the overlapping of individual bond eigenfunctions, which itself reflected a
greater density of charge concentrated along that particular direction. The
"remarkable" simplification Pauling had been searching for consisted of
working just with the angular component of the atomic wave functions. For
compounds of hydrogen and elements from the first row of the periodic
system, only s and p eigenfunctions figured in the formation of stable
bonds.
Pauling also assumed implicitly that s and p eigenfunctions did not
change during bonding and thus explained the bond angle of the water
molecule. But, according to Pauling, sometimes change in quantization
occurred, producing novel results. He gave a loose criterion for the change
of quantization: when the bond energy exceeded the s - p separation, then
s - p quantization broke. Carbon satisfied the criterion. Pauling proved that
the four best bond eigenfunctions that could be formed had a maximum
value along lines making tetrahedral angles (109º) with each other.
By applying the rules for the electron-pair bond Pauling removed the
apparent incompatibility between chemistry and quantum theory. One more
step had been taken in the reconciliation between the physicist's and the
chemist's conceptions of the atom. This time the physicist's picture was
modified, by the new idea that quantization could be broken under certain
conditions. Furthermore, Pauling's approach provided valuable insights for
the case of polyatomic molecules too complex to be treated on a rigorous
quantum mechanical basis. The fluidity of Pauling's style and the clarity of
his explanations made it relatively easy for interested chemists to take the
first steps into a territory largely unknown to them.
(126)
1931: Another valence theory
Mulliken's papers rarely shared the clarity of presentation of Pauling's.
Seldom able to restrain himself from discussing the whole story, he often
did not distinguish between crucial points and minor details. Until 1929, he
published in journals read mainly by physicists and it took him some years
to realize the importance of writing for a chemical audience. The year
following Pauling's publications of 1928, Mulliken presented at the spring
meeting of the American Chemical Society a review article on "Band
spectra and chemistry."
(127)
There, Mulliken summarized the theory of band
spectra with special emphasis on the applications to chemistry, particularly
valence theory. He explained his ideas on molecule formation together
with Heitler and London's different considerations of the same problem.
This time Mulliken's paper was a model of clarity.

Raymond T. Birge
1887-1980
Photo © National
Academy of Sciences, originating from here.
|
Birge was overjoyed by the paper. It proved to him that Mulliken could
indeed write a "simple and clear" article if he wanted to. He classified it
as the "best simple, yet comprehensive, introduction to band spectra that
has yet been written by anyone, in any language." It contained all the
information that a chemist needed to know, but omitted all unnecessary
details and complications. With such articles, Mulliken would reach a larger
audience, composed of specialists as well as non-specialists. This, Birge
told him, was one of the tasks a scientific innovator should tackle, and
perhaps the most important task Mulliken should consider at this stage of
his career:
(128)
You certainly know more about band spectra than anyone else in the world,
and now the important thing is for you to give out your knowledge in a way
that will enable others also to understand it.
Your Chemical Review article
shows that you can do this better than I can, while your Supplement article
[ref. 41] shows that you do not try to make things clear,
and in part,
non-mathematical, when you are trying to write a serious article.
If you write a
book, I hope you will write a simple, more or less non-mathematical
account
of each part, and then put all the details and abstruse things into
appendices,
even if the latter occupy more than half the book. Only in that way will
there be any sale, I think.
|
By 1931, when Pauling's first paper on "The nature of the chemical
bond" came out, Muhhiken recahled Birge's friendly advice. Having completed
the trilogy on the "Assignment of quantum numbers to electrons in
molecules" two years previously, he realized that his preliminary ideas on
valence theory were buried amidst information on band spectra, in a journal
seldom read by chemists. A presentation especially devoted to them was
timely; Mulliken presented it at the end of March 1931, at the meeting of
the American Chemical Society. Mulliken confided to Birge that he was
preparing for "an attack on the Heitler and London valence theory" and
reiterated his belief that "one can understand chemical binding decidedly
better, and more intimatehy, by a consideration of molecular electron
configurations than by Heither and London's method."
(129)
Birge had realized
that certain of Heitler and London's statements did not agree with spectroscopic
evidence (like the impossibility of having HeH or He2+,
and thus worried about the compatibihity between the spectroscopic
evidence for the
analogies between atoms and molecules and chemical knowledge of
saturated and unsaturated bonds and electron pairs.
(130) Mulliken too had
decided that there were important flaws in Heitler and London's theory:
(131)
It is becoming clear that the London valence theory is really not so good,
just
happens to agree with chemical theory. I mean, the ⇔ [pairing] of
electron is
not the real essential thing. It is that the molecular electron configuration
of
lowest energy quite usually (not always) happens to have the electrons
all ⇔ ...
His idea of ⇔ being all-important was a far-too-large
generalization.
Mulliken prepared to criticize their theories publicly and restate his own
bold considerations on valence theory. Convinced of the "arbitrariness"
and "superfluity" of concepts such as valence, valence bonds, and bonding
electrons, he intended to prove the fragility of some of the strongest pillars
of chemical science. To build them more firmly he outlined the guiding
assumptions and basic concepts of a new valence theory, which dispensed
with the cherished classical tenets.
(132)
Lewis had defined the valence bond as a pair of electrons held jointhy
by two atoms. According to Mulliken, this definition implied that pairs of
electrons acted as a sort of interatomic glue binding the atoms together, and
thus could be called bonding electrons; the remaining unpaired electrons
might be called non-bonding electrons. But Mulliken's work on band spectra
led him to conclude that, besides bonding and non-bonding electrons,
there were electrons that actively opposed bonding. Mulliken called these
anti-bonding electrons. During molecule formation, anti-bonding electrons
had to be promoted to orbits of a larger principal quantum number n, in
order to satisfy the Pauli exclusion principle. This tripartite classification
implied a continuously varying bonding power of electrons, which Mulliken
had previously defined in terms of energy relations (or alternatively in
terms of distance relations). Thus, the "problem of valence is really one of
energy relations." If there were rules to calculate the energies of the
possible molecular orbits, and to correlate them with the energies in the
associated atoms, then the "rules of valence should follow automatically."
(133)
The absence of such rules limited the scientist to qualitative
reasoning and approximate calculations. Mulliken relied largely on empirical
spectroscopic data to calculate and predict energies of atomic interactions
and molecular constants.
Mulliken presented a qualitative analysis of the formation of the
H2+ ion
and the H2 molecule in terms of energy. When the H atom and the
H+ ion
approached each other, the electron orbits deformed and tended to surround
both nuclei. In the process, the 1s atomic electron became more firmly
bound. He represented the formation of the ion by
H(1s) + H+ --->
H2+ (1sσ, 2Σg),
which meant that the atomic orbit 1s was changed into a molecular orbital
1su. In this case the energy of binding in the molecular orbit
outweighed
the energy of repulsion of the two nuclei, and the electron acted as a bonding
electron. Repulsion was represented by
H(1s) + H+ ---> H2+ (2sσ, 2Σu).
In this case, the energy of promotion, together with the nuclear repulsion,
caused the atom and ion to repel each other at all distances, and the electron
was anti-bonding.
Two similar situations occurred when two atoms of hydrogen
approached each other, represented as follows:
H(1s) + H(1s) ---> H2 (1sσ2, 1Σg),
H(1s) + H(1s) ---> H2 (1sσ 2pσ, 3Σu).
In the first mode of interaction, the two electrons entered the 1sσ
state and
acted as bonding electrons, forming a stable hydrogen molecule. In the
second mode of interaction, one of the electrons reached a 2pσ orbit and
the energy of promotion outweighed the increased binding energy of the 1s
electron, and, usually, no molecule formed. However, Mulliken noted once
again that, contrary to Heitler and London's claim, there might be a considerable
attraction at large distances, leading to the formation of a relatively
stable molecule, even though at smaller distances the large energy of promotion
led to a strong repulsion between the two atoms.
At this point Mulliken made a crucial distinction between Heitler and
London's method and their valence theory. Their method had great
potential in principle, if not always in practice. Yet, for Mulliken, it
presented a serious drawback: it "fails to give a detailed insight into the
nature of the changes which take place in the electron orbits when the
atoms come together." (134)
But, above all, Mulliken objected not so much to
their method as to their valence theory.
In Heitler and London's spin theory of valence, the valence bond
required a symmetrical relation in the position coordinates and an
antisymmetrical relation in the spin coordinates between two electrons
belonging to
two separate atoms. Mulliken could not disagree more with this explanation
of chemical bonding. The emphasis on the pairing of spins, or for that
matter on the pairing of electrons, was misleading because it pointed to a
purely "incidental" phenomenon and concealed something "more fundamental."
(135)
The case of the H2+ ion,
with only one electron, illustrated that
pairing was not relevant to the analysis of molecule formation and further
suggested that "we should regard a single bonding electron as the natural
unit of bonding, an anti-bonding electron as a negative unit.
(136)
The effect of a symmetrical relation in the positions of two electrons,
with the concomitant antisymmetrical relation in their spins, was "to make
the electrons keep on the average closer together than they otherwise
would... hence, unless other indirect effects are important, a symmetrical
relation increases the energy of repulsion of the electrons and so the total
energy, while an antisymmetrical relation decreases it." For Mulliken, the
presence of unpaired electrons with antiparallel spins and their subsequent
pairing in molecule formation was just a "convenient indicator" of valence
and the formation of valence bonds, but did not "hit the nail on the
head."
(137)
Heitler and London defined the valence V of an atom as V=2S, S denoting
the spin of the atom. The valence of an atom equalled the number of
unpaired electrons; since each electron had a spin quantum number of 1/2,
if there were n unpaired electrons the resultant spin would be S = n/2, so
that n = 2S = V. The theory further implied that when an atom did not have
any unpaired electrons, n = S = V= 0, and it could not form any bonds. This
rule was bluntly contradicted by evidence from band spectra, which showed
that molecules such as HeH and He2+
could form. According to Heitler and
London, the helium atom could not bond to a hydrogen atom because the
two ls electrons were already paired in the helium atom. However, when
the hydrogen atom approached the helium atom, the 1s electron of the
hydrogen atom could be promoted to a 2pσ orbit. In general, the large
promotion energy causes the two atoms to repel each other. However, in
analogous cases, such as when a helium atom and a helium ion approach, a
physically stable molecule could be formed:
He(1s2) + He+(1s)---> H22+ (1sσ2 2pσ).
In this process, confirmed by spectroscopy, the two 1sσ bonding
electrons outweighed the anti-bonding 2pσ electron. The existence of
molecules such as HeH or He2+
illustrated the arbitrary nature of valence
and "the impossibiiity of accepting it as corresponding to an always sharply
definabie, whole number property of atoms."
(138) Taking advantage of
his command, and deep understanding, of spectroscopic data, Mulliken
made a distinction between chemical and physical stability. HeH and
He2+
are not chemically, but physically stable. Mulliken suggested that chemical
binding could best be understood in terrms of quantum numbers of individual
electrons and their changes in moving from the united atom to the
molecule, as well as in tenns of the energy relations resulting when going
from the separated atoms to the molecule. The strength of the chemical
bond related to the electronic quantum numbers. Mulliken boldly proposed
to dispense altogether with classical valence theory, which he called the
"atomic point of view" and to adopt instead a new "molecular point of
view:"
(139)
In the "molecular" point of view advanced here, the existence of the
molecule as a distinct individual built up of nuclei and electrons is
emphasized, whereas according to the usual atomic point of view the
molecule is regarded as composed of atoms or of ions held together by
valence bonds. From the molecular point of view, it is a matter of secondary
importance to determine through what intermediate mechanism (union of
atoms or ions) the finished molecule is most conveniently reached. It is
really not necessary to think of valence bonds as existing in the
molecule.
Pauling develops his program: The nature of the chemical bond
A comprehensive theory of the chemical bond based on the concept of
resonance emerged out of Pauling's series on "The nature of the chemical
bond," which he finished by 1933.
(140)
The concept of resonance had played
a fundamental role in the discovery of the hybridization of bond orbitals
and the one-electron and the three-electron bond, and the discussion of the
partially ionic character of covalent bonds in heteropolar molecules. Furthermore,
resonance explained in "an almost magical way" the many puzzles
that had plagued organic chemistry.
(141)
Resonance linked Pauling's new
valence theory and the classical structural theory of the organic chemist,
which Pauling classified as "the greatest of all theoretical constructs."
Resonance - originally a physical concept - now became absolutely crucial in
the formulation of a chemical theory:
(142)
The theory as developed between 1852 and 1916 retains its validity. It has
been sharpened, rendered more powerful, by the modern understanding of the
electronic structure of atoms, molecules and crystals; but its character
has not
been greatly changed by the addition of bond orbitals, the theory of
resonance, partial ionic character of bonds in relation to
electronegativity, and so
on. It remains a chemical theory, based on the tens of thousands of chemical
facts, the observed properties of substances, their structure, their
reactions. It
has been developed almost entirely by induction (with, in recent years, some
help from the ideas of quantum mechanics developed by the physicists). It is
not going to be overthrown.
By 1935 he felt that he had acquired an "essentially complete understanding
of the nature of the chemical bond."
(143)
Always eager to get his
contributions recognized quickly by his peers, Pauling used all communication
channels in order to reach as many people as possible. In 1935 he published
in collaboration with E.B. Wilson the Introduction to quantum
mechanics with applications to chemistry. Addressed to chemists, experimental
physicists, and beginning students of theoretical physics, the book
did not presuppose much mathematical background on the part of its
readers. It became extremely popular, even among those familiar with quantum
theory.
(144)
During his non-resident tenure at Cornell University in the fall semester
of 1937, Pauling reorganized for publication all his material on the chemical
bond. The book, dedicated to Lewis, appeared in 1939 and sold so well
that another edition came out the following year. Lewis was overjoyed. "I
have returned from a short vacation for which the only books I took were a
half dozen detective stories and your 'Chemical Bond.' I found yours the
most exciting of the lot. I cannot tell you how much I appreciate having a
book dedicated to me which is such a very important contribution. I think
your treatment comes nearer to my own views than that of any other
authors I know and there are very few places where I could possibiy
disagree with you; and those perhaps because I have not thought about the
thing sufficiently."
(145)
Pauling presented a coherent treatment of the chemical bond that
appealed to chemists because of its frequent reliance, as he mentioned, on
the "chemists' intuition" and the use of existing experimentai data to
predict or explain other experimental data. Though he repeatedly stressed
that only quantum mechanics could explain the chemical bond, he used a
bare minimum of mathematical formulations. He did not aim at "proving"
theorems, but rather at "devising" rules that did not follow in any rigorous
way from more general principles; yet they seemed reasonable, they were
partially justified by quantum mechanics, and, most significantly, they
produced results. As Pauling has often said, it was a pragmatic approach to
chemistry, a semi-empirical treatment of its central problems.
Throughout his career, Pauling published on valence theory with almost
total disregard for alternative theories. He never referred publicly to the
molecular orbital approach. However, in 1936 and 1937, Pauling and
George Wheland worked on a book entitled Quantum mechanics of organic
molecules in which they planned to compare the valence bond and molecular
orbital methods.
(146)
Pauling was supposed to prepare the chapters on the
evaluation of both methods. He never finished his share in the project. He
drifted toward applications to larger molecules of biological interest.
(147)
Later on, Pauling tried to revive the project, regretting his "dilatoriness" in
pushing the book forward in the first place, but by then too much revision
and restructuring would have been needed.
(148)
|
|
 George Wheland
George Wheland
1907-1976?
Photo and ©
J.D. Roberts
|
Apparently only Pauling could have stated his rivals' views with enough
power to keep them alive. That in any case is what one gathers from
Joseph Mayer's review of The nature of the chemical bond:
(149)
It is unfortunate that this treatise will almost certainly tend to fix,
even more
than has already been done by the author's excellent papers, the viewpoint of
most chemists on this, and only this one, approach to the problem of the
chemical bond. It appears likely that the H - L - P - S method will entirely
eclipse, in the minds of chemists, the single electron molecular orbital
picture, not primarily by virtue of its greater applicability or
usefulness, but
solely by the brilliance of its presentation.
Mayer considered that another book on the same subject, using the same
examples but emphasizing aIternative methods, would help break the monopoly,
but be predicted that it would be hard to emulate the clarity and
simplicity of Pauling's presentation. Such a book was never written. Even
though Mulliken strived to explain and publicize his alternative program,
Mayer's review is witness to his limited success.
Pauling knew of the difficulties inherent in the understanding of such an
unfamiliar concept as resonance. He took pains to explain its meaning as
clearly and succinctly as possible. In The nature of the chemical bond he
discussed the meaning of quantum mechanical resonance as well as the
meaning of the chemical resonance of molecules among several valence
bond structures and its significance for chemistry. It was very easy for
chemists to misinterpret the significance of the concept of resonance.
Pauling pointed out that there existed an "element of arbitrariness" in the use
of resonance as a result of the choice of the canonical structures in the
system, but he argued forcefully that "the convenience and usefulness of the
concept of resonance in the discussion of chemical problems are so as to
make the disadvantage of the element of arbitrariness of little
significance."
(150)
This, as he repeatedly mentioned, was his criterion for
building chemical theories - a specification of Bridgman's operationalism in
chemistry.
(151)
Pauling and Wheland, who more than anyone else worked to extend
resonance theory to organic molecules, could not agree over the interpretation
of resonance. In his book The theory of resonance and its applications
to organic molecules (dedicated to Pauling), Wheland argued that resonance
was a "man-made concept" in a more fundamental way than most other
physical theories. Wheland countered the widespread view that resonance
was "a real phenomenon with real physical significance," which he
classified as one example of the nonsense to which organic chemists were
especially prone.
(152)
Pauling and Wheland agreed on neither the
methodological nor the ontological status of resonance.
As Wheland put it,
(153)
What I had in mind was, rather, that resonance is not an intrinsic property of
a molecule that is described as a resonance hybrid, but is instead something
deliberately added by the chemist or the physicist who is talking about the
molecule. In anthropomorphic terms, I might say that the molecule does not
know about resonance in the same sense in which it knows about its weight,
energy, size, shape, and other properties that have what I call real physical
significance. Similarly... a hybrid molecule does not know how its total
energy is divided between bond energy and resonance energy. Even the double
bond in ethylene seems to me less "man-made" than the resonance in
benzene. The statement that the ethylene contains a double bond can be
regarded as an indirect and approximate description of such real properties as
interatomic distance, force constant, charge distribution, chemical
reactivity,
and the like; on the other hand, the statement that benzene is a hybrid of the
two Kekulé structures does not describe the properties of the
molecule so
much as the mental processes of the person who makes the statement.
Consequently, an ethylene molecule could be said to know about its
double bond,
whereas a benzene molecule cannot be said, with the same justification, to
know about its resonance... Resonance is not something that the hybrid
does, or that could be "seen" with sufficiently sensitive apparatus, but is
instead a description of the way that the physicist or chemist has
arbitrarily
chosen for the approximate specification of the true state of affairs.
Pauling could not disagree more. For him, the double bond in ethylene
was as "man-made" as resonance in benzene and he believed that in that
way the younger generation of chemists, who had grown familiar with resonance
theory, would handle both molecules. There was no essential
difference between resonance theory and the classical structural theory of
organic chemistry. Pauling summarized their divergent viewpoints by saying
that Wheland seemed to believe in a "quantitative difference" in the
"man-made" character of both theories - resonance theory being "more
man-made" than ordinary structure theory - but he, himself, could not find
such a difference. He asserted that Wheland disparaged resonance theory
by overemphasizing its "man-made character."
(154)
Wheland conceded that
resonance theory and classical structural theory were qualitatively alike and
added that "the qualitative identity of the theories is doubtless the reason
why, as an organic chemist, I prefer the resonance to the molecular-orbital
approach," but he still defended, contrary to Pauling, the "quantitative
difference" between the two."
(155)
When I say, for example, that benzene is a hybrid of the Kekulé
structures, I
at once introduce all the human elements that are implied by the term
"Kekulé structure," and that are, in fact, implied whenever
I speak of any
valence-bond structure of any molecule; in addition, however, I am also
specifying which particular one of the several available mathematical
procedures I have arbitrarily, and merely for reasons of convenience,
decided to
adopt. The first group of "human elements," that are unavoidable in any use
of the structural theory, forms an essential part of an attempt to describe a
system which is physically real or, at any rate, which can be imagined to be
physically real; on the other hand, the statement that I am using the
resonance
approach says as much about me as it does about the molecule... I feel that
there is a real quantitative difference since the resonance theory
contains not
only all the man-made features of the structural theory but also some
additional ones (such as its specific quantum-mechanical background and
language); these additional features are completely absent from the structural
theory and, moreover, they can be replaced by other equally man-made
features (as, for example, in the molecular-orbital approach) without directly
affecting the structural theory.
Wheland viewed their disagreement as a result of different value-
judgements on what he classified as philosophical rather than scientific
rnatters. Nevertheless, their differences over resonance theory and classical
structural theory depended on their different assessments of the role of
alternative methods to study molecular structure. Wheland equated resonance
theory to the valence-bond method and viewed them as alternatives
to the molecular-orbital method. Pauling conceded that the valence-bond
method could be compared with the molecular-orbital method, but asserted
that the resonance theory was largely independent of the valence-bond
method. For Pauling the theory of resonance was not merely a computational
scheme. It was an extension of the classical structure theory and
shared with its predecessor the same conceptual framework; if one accepted
the concepts and ideas of classical structure theory one had to accept the
theory of resonance. And how could any one reject their common conceptual
base if they had been largely induced from experiment? Pauling
continued:
(156)
I think that the theory of resonance is independent of the valence-bond
method of approximate solution of the Schrödinger wave equation for
molecules. I think that it was an accident in the development of the sciences
of physics and chemistry that resonance theory was not completely formulated
before quantum mechanics. It was, of course, partially formulated
before quantum mechanics was discovered; and the aspects of resonance
theory that were introduced after quantum mechanics, and as a result of
quantum mechanical argument, might well have been induced from chemical
facts a number of years earlier.
This discussion with Wheland prompted Pauling to publicize his
epistemological position. More than the question of the artificiality of the
resonance concept, to which he alluded briefly in his Nobel lecture,
(157)
he now emphasized his views on theory building. He quickly prepared a
revised version of the arguments brought about in the discussion with Wheland.
It appeared first in Perspectives in organic chemistry and later in the
third edition of The nature of the chemical bond.
(158)
Pauling titled his manifesto
"The nature of the theory of resonance." He argued that the objection
concerning the artificiality of concepts applied as well to the resonance
theory as to the classical structure theory. To abandon the resonance theory
was tantamount to abandoning the classical structure theory of organic
chemistry. Were chemists willing to do that? According to Pauling, chemists
should keep both theories because, as chemical theories, they possessed
"an essentially empirical (inductive) basis:"
(159)
I feel that the greatest advantage of the theory of resonance, as compared
with other ways (such as the molecular-orbital method) of discussing the
structure of molecules for which a single valence-bond structure is not
enough, is that it makes use of structural elements with which the chemist is
familiar. The theory should not be assessed as inadequate because of its
occasional unskillful application. It becomes more and more powerful, just
as does classicai structure theory, as the chemist develops a better
and better
chemical intuition about it... The theory of resonance in chemistry is an
essentially qualitative theory, which, like the classical structure theory,
depends for its successful application largely upon a chemical feeling that is
developed through practice.
Mulliken's program: What are the electrons really doing in
molecules?
Mulliken extended his program to polyatomic molecules with a series of
fourteen papers entitled "The electronic structure of polyatomic molecules
and valence," which spanned the years 1932 - 35. The series appeared in
the Physical Review and, after the fourth paper, in the newly created
Journal of Chemical Physics, which some claim was founded just to present
Mulliken's papers.
(160)
The first paper contained a brief outline of Mulliken's
proposal.
(161)
Originally intended as a harsh critique of the Slater-Pauling
approach, the letter-turned-paper underwent considerable modifications as a
result of discussions with Van Vleck. In the final version a softened criticism
of alternative approaches was relegated to a long "added in proof."
The correspondence with Van Vleck shows how matters stood:
(162)
It was very nice of you to write me so extensively, and very helpful in
inducing me to get back as far as possible into the Pauling-Slater frame of
mind... I think
One-electron Ψ's in Molecule...
----------------------------------------------------------------
,

Localized binding of Pauling and Slater
--->
Deeper Understanding of Molecular Structure and Valence
------------------------------------------------------------------------------
Conventional valence theory with electron-pair bonds
In other words, I think the present method brings one much closer to a real
insight into the electronic structures of finished molecules, enabling one to
understand both the majority of molecules which obey ordinary valence rules
pretty well and the minority which do not. Whereas the Slater-Pauling theory
aims at and succeeds in getting a poorer
approximation which fits merely the
ordinary phenomena of homopolar valence. Of course the Slater-Pauling
theory, using the Heitler-London methods, offers a
method of calculation,
which is important. But I think a deeper qualitative insight into molecular
structure (which incidentally visualizes polar and non-polar intermediate
cases, not as linear combinations, but directly) is also important...
I have assumed the tetrahedral structure only as an empirical fact,
Although remarking that it is not surprising. I now agree that their
results on
directed valence in H20 and NH3 are probably O.K.
I feel pretty sure (as
Hund seems to) that their explanation of the double bond and its properties is
not correct... Slater pointed out a while ago that one needs to mix in quite a
large percentage of the polar H+ + H-
with the Heitler-London H + H to get
the approximation to the ground state of H2
by using atomic Ψ's. This is an
example showing how molecular Ψ's which are not localized pairwise in
regions of bonds can have advantages over Heitler-London pairs.
Mulliken started freely using the language of quantum mechanics in
these papers. Now, he associated an atomic orbital with the motion of an
electron in the field of a single nucleus and of other electrons, and a molecular
orbital with the motion of an electron in the field of two or more nuclei
and of other electrons. He had thought to use many-centered ("nonlocalized")
molecular orbitals, even though other types of orbitals could be
used, like two-centered ("localized") orbitals.
(163)
The correlation diagram
implied that for large internuclear separations a linear combination of
atomic orbitals could approximate a molecular orbital. Although he considered
it a partial departure from his original program, Mulliken followed
Lennard-Jones's suggestion for diatomic molecules and extended it to the
case of polyatomic molecules. He represented unshared (non-bonding)
electrons by atomic orbitals, and shared (bonding or anti-bonding) electrons
by molecular orbitals, which were quite independent from atomic orbitals:
"The present method of thinking in terms of the finished molecule, used
already by Lewis in his valence theory, avoids the disputes and ambiguities,
or the necessity of using complicated linear combinations, which arise if
one thinks of molecules as composed of definite atoms or ions."
(164)
After defining his terms, Mulliken presented a semi-historical survey of
some of the most recent valence theories with the aim of showing that
"there are no compelling reasons, either empirical or theoretical, for placing
primary emphasis on electron pairs in constructing theories of valence.
[The author] hopes thereby to remove possible objections to the present
method based on its lack of such emphasis."
(165)
He then compared the
valence theories of Lewis, Heitler and London, and Slater and Pauling by
determining the different weight attributed in each theory to three theoretical
components: (A) Each nucleus tends to become surrounded by a set of
closed shells of electrons (group of eight, or octet); (B) Shared electrons in
covalent bonds are localized between the nuclei they glue together; (C) A
chemical bond usually consists of a pair of electrons tied to each other
(group of two).
Lewis's theory emphasized component (C) relative to components (A)
and (B). In Heitler and London's spin theories of valence, component (C)
had priority over (B) and (A). Slater and Pauling stressed component (B)
relative to (C). Relative to Lewis's, the H-L-P-S theory implied more
strongly that each electron comes from each atom. To stress this difference,
Mulliken suggested calling the H-L-P-S theories electron-pairing theories
in contrast to Lewis 's electron-pair theory.
On the other hand, the theory of molecular orbitals emphasized
component (A), adopted component (B) in a generalized form (many-centered,
non-localized molecular orbitals), and considered component (C) an
incidental characteristic of chemical combination. Mulliken believed that
the H-L-P-S approach put an unjustifled emphasis on the role of electron-
pairs and electron-pairing. He further argued that, relative to the electron-
pair bond of H-L-P-S, the concept of molecular orbitals presented a
number of advantages: it did not assume that two electrons were necessary
to form a chemical bond; bonding molecular orbitals could have any degree
of polarity corresponding to different degrees of sharing of electrons
between the nuclei; and bonding molecular orbitals were not restricted, like
electron-pair bonds, to just two electrons. He also claimed that the concept
of bonding molecular orbitals was more general, more flexible, and more
natural than the concept of electron-pair bonds, even though "the electron-
pair bond method may for many problems be more adapted to quantitative
calculations than the present method.
(166)
Three years after this thorough restatement of the guiding assumptions
of his program, Mulliken still felt that the theoretical framework of his
unconventional valence theory was not understood clearly and he attempted
once again to clarify the situation. He sent a copy of the manuscript to Van
Vleck:
(167)
There is nothing fundamentally new in it, but I feel it is called for to
clarify
the situation. I have always had in mind the idea of a "conceptual scheme"
to be compared with empirical data, but seem never to have stated this very
clearly. The conceptual scheme using "natural" or "real" or "best" (even
though of not known exact form) molecular orbitals must represent a better
approximation than the use of rough LCAO [linear combination of atomic
orbitals] orbitals."
Two different descriptive chemical theories provided alternative methods
for the assignment of molecular electron configurations, one of them relying
entirely on the use of atomic orbitals, the other using molecular orbitals of
some sort to describe shared electrons:
(168)
The first method follows the ideology of chemistry and treats every molecule,
so far as possible, as composed of definite atoms or ions. The electron
configuration is then the sum of the configurations of these atoms or
ions.... [This method] has had notable success as a qualitative conceptual
scheme for interpreting and explaining empirical rules of valence and in
semiquantitative, mostly semiempirical calculations of energies of formation
and other properties of molecular electronic states, particularly the normal
states.
Departing from chemical ideology, the second method treats each
molecule, so far as possible, as a unit.... It is the writer's belief
that the
present... [method of non-locaiized molecular orbitals] may be the best
adapted to the construction of an exploratory conceptual scheme within
whose framework may be fitted both chemical knowledge and data on electron
levels from molecular spectra. A procedure adapted to a broad survey
and interpretation of observed relations is here aimed at, rather than
(at first)
one for quantitative calculation, which logically would follow later. Given an
observed molecule or ion of known shape and size, what is its electronic
structure in terms of an electron configuration using, in general,
non-iocalized orbitals for shared electrons?
According to Mulliken it was utterly unfair to criticize the molecular
orbital theory on the basis of the poor results obtained by the use of a
rough approximation such as the LCAO representation of a molecular
orbital: "While the LCAO type of approximation is very simple and convenient
as a qualitative guide, it is in no way an essential part of the present
method. Especially it is not essential to the qualitative conceptual scheme
of the latter."
(169)
The molecular orbital theory was also criticized on the
ground that it neglected the interactions between electrons. Mulliken
argued that "their qualitative inclusion has always formed a vital part of
the method of molecular orbitals used as a conceptual scheme for the
interpretation of empirical data on electronic states of molecu1es.... For
instance it is precisely because of such interactions that the present method
was able to offer the first, and a very simple and satisfactory, qualitative
explanation of the paramagnetism of the oxygen gas." In evaluating his
valence theory, Mulliken challenged people to distinguish between its
conceptual scheme and the available computational methods. Mulliken summarized
the divergence between his and Pauling's viewpoints in a lecture at the
adversary's camp:
(170)
It may seem that I am bringing coals to Newcastle. I can only hope,
however, that the coals I bring may be sufliciently different from the
excellent
local product to make them interesting... My point of view in these lectures
may be defined by asking "what is a molecule?" To chemists generally a
molecule is an aggregate of atoms or ions, held together by valence forces,
whose laws are of primary interest. The point of view which I shall follow is
one which is perhaps more natural to physicists, and in which molecules are
regarded primarily as aggregates of nuclei and electrons. Those aggregates
whose lowest states happen to be particularly stable in free competition with
other possible aggregates are the chemical molecules. In pursuing this point
of view it is advisable, however, at the outset to make a partial concession
to
the chemical point of view, so as to regard molecules as aggregates of atom-
cores or atom-ions and electrons, rather than just of bare nuclei and
electrons. In other words, a certain number of electrons, in general,
most of the
electrons, may for all practical purposes be assigned definitely to specific
nuclei so as to form atom cores or ions, leaving only a generally smaller
number to belong to the molecule as a whole.
Mulliken objected to the oversimplification he saw in the phrase "The
nature of the chemical bond." For him, the chemical bond had many
natures. Besides, he did not want to comrnit himself to the conventional
view' according to which "atoms were still atoms when they have formed
molecules." In the manner of Gertrude Stein, he would rather say "a
molecule is a molecule is a molecule."
(171)
However distinct Mulliken and Pauling's approaches to valence theory
looked, important similarities existed between them. Both provided theoretical
frameworks for the explanation - not the prediction - of molecular structure.
The structure of molecules was determined by experimental methods
such as X-rays, electron diffraction, or band spectra, and the theory did not
provide a basis for challenging the experimental evidence, however
questionable it might appear. Another common characteristic of both approaches
was the emphasis on solving problems of molecular structure and bonding
through the development of semi-empirical methods. Chemical arguments
and empirical data figured prominently in conjunction with quantum theory.
By 1937, Mulliken had realized that his conceptual scheme had to be
supplemented by semi-empirical methods in order to get practical results:
"We depend wholly on the quantum mechanics only in a very few cases. In
more complicated cases, we make partial use of quantum mechanics in the
form of qualitative principles or rules. Into this qualitative theoretical
framework we then try to fit all relevant experimental data. By this semi-
empirical method, we are able to reach many conclusions concerning
electronic structures of molecules. In doing this, we may use many kinds of
physical and chemical data, including spectroscopic data."
(172)
For once
Pauling might have agreed with Mulliken. For Pauling, molecular problems
and the chemical bond called for a "half theoretical-half empirical"
approach, which he had adopted in extending Heitler and London's method
to molecules more complex than the hydrogen molecule: "Their efforts to
extend the theory to more complicated molecules were not very successful.
My method has a greater empirical content than the others, but the same
rigorous quantum mechanical basis."
(173)
But not everyone involved approved the new rules of the game.
Heitler and London versus Pauling and Mulliken
Apart from the letters Heitler and London exchanged in late 1927 about
the possibilities offered by group theory, they made no contact until 1935,
when they started frantically writing to each other. They were both in
England, having resigned their positions after the Nazi decrees of April 1933.
Heitler was at the Department of Physics of the University of Bristol,
London at Clarendon Laboratory in Oxford. The publication of the papers by
Slater, Pauling, Van Vleck, and Mulliken prompted the renewal of their
correspondence.
This correspondence describes their tense search for the means to consolidate
their theory when the Americans appeared to be taking over the field
of quantum chemistry and reflects the different styles of their environments.
Faithful to the Göttingen spirit, Heitler was "more mathematical," while
London continued in the Berlin tradition of theoretical physics with its
inclination toward intuitive proposals. They discussed the possibility of
writing an article in Nature to present their old results and to add the
activation of spin valence and the possibility of a bond that did not depend
on spin saturation. "That [possibility, Heitler wrote] is what I meant in a
past note - vaguely and wrongly - with the term orbital valence."
(174)
Heitler believed that Slater and Pauling adopted the correct principles
and he was quite sympathetic about the direction of their research, even
though it did not lead straight to a series of results. He thought a polemic
against them unjustified: "I simply find that the importance of this theory
has been monstrously overrated in America."
(175)
For the first time Heitler and London expressed doubts about the character
of the attractive forces. It was conceivable that these forces were not
only due to spin. Other attractive forces of the same order of magnitude
that did not follow from their original theory of spin valence might exist.
These forces would not originate only from the directional degeneracy of
the ground state, and could result in the formation of a molecule if there
were also spin valences. They admitted to one another that they did not
know much about what happened when more than two atoms were near
each other, since the mechanism of the problematic forces differed from
that of spin valences:
(176)
The next question is whether one should consider these forces, that are added
to our original ones, as valence forces. Well, the chemists undoubtedly do it,
since they name, or, rather, they named in this way whatever gives molecules
(in contrast to the v.d. Waals forces and the pure ionic molecules). This is
exactly our job. To say that there are also other forces of molecule
formation, except our old ones, and which phenomena of chemical
valence depend on those, and that our old scheme can be extended.
Heitler felt that there had been no attack against them by the Americans,
except for the case of the oxygen molecule whose paramagnetism they
could not explain:
(177)
The nucleus of our theory is the spin valence and that our theory is the only
one that explains the mechanism of repulsion in a qualitatively exact manner.
It is needless to write this since we surely agree on that. You could perhaps
include the above discussion under the title:
Delineation of completeness (so
much of theory as well as of the chemical notion of valence that corresponds
to theory). In any case, we should stress that the extension could be realized
on the basis of our theory and, substantially, it includes whatever one could
wish (this last thing only as a footnote for us). It is ridiculous even from a
quantitative point of view.
London's answer was not a compliment to chemists:
(178)
The word "valence" means for the chemist something more than simply
forces of molecular formation. For him it means a substitute for
these forces
whose aim is to free him from the necessity to proceed, in complicated cases,
by calculations deep into the model. It is clear that this remains wishful
thinking. Also the fact that it has certain heuristic successes. We can, also,
show the quantum mechanical framework of this success... the chemist is
made out of hard wood and he needs to have rules even if they are
incomprehensible.
They came gradually to realize their isolation; Heitler did not agree with
London's claim that their theory was "fought by the most unfair and secretive
means."
(179)
It may be true for some people in America. Not all people, however, are
rascals (e.g., I would not believe it for Van Vleck), but only silly
and lazy. And
we should accept that our theory was quite complicated. I would gladly like
to look at the books of Sidgwick and Pauling. I cannot get them here.
Heitler thought that London was being paranoid. London replied that
Heitler should look up the assessment of their work by Mulliken and R.
Kronig, and "judge for yourself whether we are neglecting something or
not, when we leave unanswered these kinds of distortions. And they are
not at all isolated cases."
(180)
Mulliken's main objection was methodological. The approach of Heitler
and London required long calculations in order to make quantitative predictions,
but "qualitative predictions can usually be made much more easily
by a consideration of electron configurations of atoms and molecules."
(181)
These kinds of pronouncements deeply angered London. He did not mind
there being a theory "superior" to his own, but one had to play the game
according to the rules and not devise new rules along the way. He disagreed
with the practice whereby experimental data was used as part of theory
construction. So much the worse when these rules were nothing but
rationalizations
of experimental data. Some years later he would be furious when he
thought that Landau was doing the same thing in superfluidity.
Still, Mulliken had attempted to give Heitler and London due credit in
1928. He considered their joint work and the subsequent papers using group
theory, together with Hund's papers, as promising "at last a suitable
theoretical foundation for an understanding of the problems of valence and
of the structure and stability of molecules," and he mentioned the agreement
of some of his results with those of Heitler.
(182)
He praised London's
theory as a translation of Lewis's theory into quantum mechanical
language.
(183)
By 1933, however, he was not referring to the Heitler-London
paper, but rather to the theory of Slater and Pauling, which, together with
the molecular-orbital approach, illuminated Lewis's theory from more or
less complementary directions.
(184)
In his Nobel speech in 1966 he mentioned
the paper as merely initiating an alternative approach to the molecular
orbital method. He did not even recognize that it provided the quantum
mechanical explanation of Lewis's schema, since the "electrons in the
chemical molecular orbitals represent the closest possible quantum mechanical
counterpart to Lewis's beautiful pre-quantum valence theory."
(185)
In an
article in 1931 in Chemical Reviews, Mulliken expressed in a detailed
manner his objections to the Heitler-London method and theory, but London
curiously never mentioned it.
The appearance of Kronig's book in 1935 did nothing to alleviate
London's feeling that he and Heitler should take a strong stand against
distortions of their work.
(186)
Kronig was quite harsh toward Heitler and London
while welcoming toward Slater and Pauling. Kronig mentioned quite a few
shortcomings of the Heitler-London approach. It could not deal successfully
with atoms not in their ground state. It could not explain the
numerous compounds between oxygen, sulfur, and the halogens. The calculations
were done only to first approximation, which made many of the
results doubtful. Though Kronig recognized that the approach of Slater and
Pauling gave the same results as that of Heitler and London for atoms that
had only s-electrons outside the closed shells, Slater and Pauling had the
unquestionable advantage of interpreting the directed nature of valence
bonds in the case of the p-electrons. For the Slater-Pauling approach, "the
mathematical procedure is again a perturbation calculus starting from atoms
in the limiting case of infinite separation, but the criticism of its
applicability was not as severe as for the Heitler-London theory
since all the low-lying atomic states are taken equally into account."
(187)
At Oxford at the beginning of December 1935, Heitler read a paper by
Wheland that included this passage:
(188)
The Heitler-London-Slater-Pauling (HLSP) method... which was developed
originally by Slater as a generalization of Heitler and London's treatment of
the hydrogen molecule, was first applied to aromatic compounds by E.
Hückel, but has since been greatly simplified and extended by Pauling and
co-workers.
Heitler was vitriolic in his comment to London:
(189)
I propose in the future to talk only about the theory of Slater-Pauling of the
chemical bond, since, in the last analysis, the H2,
well now - what can this be
compared with the feats of the Americans? I am afraid that the reading of the
papers that we have voluntarily undertaken shall be the purgatorium of our
souls. If you cannot restrain me, I think, I will write a very clear letter to
this Pauling (he should give a better upbringing to his students). I think
that
you are right that we should publish in the blue journal
[National Academy of Sciences, Proceedings]. It would be
really good to write something which will mostly have those things which
they are stealing in America. Do not think I am exasperated because (in the
case of Pauling) it involves my paper with Rumer, but because of our common
cause. Your achievements disappear equally in the lies... For Van
Vleck I notice that his papers are more dignified than his report and does not
thank Slater and Pauling for nothing.
They decided to find an excuse to write to Pauling admitting that the
work of Pauling and Slater did, in fact, go beyond their original theory.
London took it upon himself to carefully read their papers: "We should
find many points where it will be evident that the passages were written in
bad faith... The best thing would be to have as an excuse a substantial
question or a criticism to Pauling's papers."
(190)
Slater's "shameless
behavior starts from 1931." In his fundamental work he claimed that the
theory of Heitler and Rumer held only when the bond energy was small
with respect to multiplet dissociation and, therefore, it had no physical
meaning. This was not correct, because Slater confused multiplet dissociation
with the separation of terms via the Coulomb interaction. Heitler, then,
made a specific proposal to London:
(191)
The local chemists, in hordes, torment me with that wretched
B2H6. It is a
typical case where there should be special reasons for bonding.
The examination
of this reason would be useful for the following reasons: 1) the
opportunity
is given to underline your view that it is possible for special forces
to
exist, but what is generally valid is the formation of pairs. 2) it would have
impressed many chemists. 3) it would let the wind out of the sails of certain
ill intentioned or silly people.
After a few weeks study, Heitler blasted Pauling as follows:
(192)
I was looking for ways to devour the so-called theory of S[later]-P[auling].
These types are so proud about something which is not so bad, but which,
under no circumstances, is so distinguished. It gives a general formula for
the bond, that corresponds to the pair bonding and the repulsion of the
valence lines. The bonding energy is additive, and the directional properties
are included. The approximation is as rough as in my semi-classical theory
(without such mathematical gurus), but it is surpassed since it includes the
directional properties. One, however, totally loses 1) the activation energy,
2) the non-additivity of the bond energy. It is needless to say that
it is fully
based on our ideas... We should not, though, make the error and regard this
work as bad or insignificant (as these people do). It is a branching from our
work, from about the point where we strictly suppose that the atoms are in
only one state... Generally, I believe that we made the mistake to leave it to
the chemists (who are nearer to this kind of work) or to types like Eyring. I
do not find, though, that our direction is not being given any attention
(apart from the details) in Europe.
As for the molecular orbital approach, Heitler thought that their basic
objection with "Hund's people" - who, both agreed, were not the bigger or
more unpleasant enemy - was not with the results. In fact, sufficient patience
with the calculations and a lot of semi-empirical considerations gave correct
results. "Nevertheless, no one could name this a general theory - much less
a valence theory - since all the general and substantive points are forever
lost."
(193)
After Born expressed his intention to write an article on the valence
bond method, Heitler and London encouraged him "since it is very difficult
for us to correct the situation with the necessary emphasis." London
reported to Born all the developments and duly informed Heitler of the
details, since he was the "sole representative of our enterprise in England."
(194)
London, in the meantime, had moved to Paris.
An article by Lennard-Jones gave the opportunity for further
clarifications of London's position. Lennard-Jones preferred the "one-
electron-orbital-bonds" and presented the version by Heitler and London
"as not so beautiful and as inadequate." London asked Born's advice on
how to get out of the quagmire:
(195)
Maybe it was a mistake that we never expressed the objections we had from
the beginning on questions of principle concerning the approach of Lennard-
Jones-Mulliken. Both of us thought it as superfluous, because we had both
"transcended" this same phase of Lennard-Jones-Mulliken in the beginning
of our observations in 1927, and we were proud when we realized that we got
the exchange degeneracy because of the similarity of the electrons. For this
reason we thought as totally evident the self-destruction of the approach by
Lennard-Jones-Mulliken, and maybe, for this reason we did not take it
seriously. I talk very often with Heitler about this lost ground and
repeatedly
we tried to find a way to make up for it. We continuously fail. We have,
undoubtedly, made a mistake by not taking seriously our competitors... The
situation had become clear since 1932 - 33, when we should have thought to
find new issues and not make enemies with our polemics.
Born decided not to publish anything about the problems of valence,
since he had not closely followed developments. But he thought it was
absolutely necessary that London and Heitler take a position and publish
something that would be accessible to chemists. He offered to ask his publisher
to start a new series in which they could be the first to write about
the chemical bond.
(196)
Heitler and London eventually realized that "in the last analysis the
pressure to do what is necessary falls on us. What is needed to keep the
more dangerous of our colleagues, those, in other words, who work with
our method, from falsifying history (Eyring, Pauling, etc.) in their place, is
a good standard book. Would you not want to write it?"
(197)
Oxford University Press encouraged Heitler to write another book,
especially after the
success of his book on radiation, and he toyed with the idea of writing one
with London about quantum mechanics and chemistry.
Then, suddenly, they gave up. London's move to Paris; Heitler's success
with his book and his work in quantum electrodynamics; London's success
with the theory of superconductivity allowed them to be gracious.
Heitler - unlike London - returned to the subject of the chemical bond in
review articles written in 1955 and 1967, and in his book Wave mechanics.
(198)
In all these writings he advocated the approach he initiated with London
and developed in their separate work. He reminded readers that the
four valences of carbon and the paramagnetism of the oxygen molecule
were explained by London and himself in terms of the non-crossing of the
potential curves.
(199)
They had shown that because of this non-crossing, the
repulsive potential of the ground state increases the
attraction owing to the
excited state by pushing down the curve for the attractive potential of the
excited state.
Why did Heitler and London never manage to co-author another article
or book? Obviously their different scientific interests after 1933, their
wanderings, and their professional insecurities played a role. Hard feelings
and misunderstandings about the way each of them started publishing in
group theory had been overcome by 1933 and so in 1935 - 36 did not undermine
a possible collaboration. The main reason that nothing then happened
was a difference in their views about the role of physics in their
approach.
(200)
Heitler's strong reductionism is evident in all his writings.
The last paragraph of his book Elementary wave mechanics with applications
to chemistry reads: "Reviewing the contents of the last three chapters
it can be said that wave mechanics is the tool for a complete understanding,
on a physical basis, of all the fundamental facts of chemistry."
(201)
He expressed the same view in later papers:
(202)
The purpose [of the early investigations in chemistry] was to understand the
phenomena of chemistry and to reduce them to laws of the newly created
atomic physics... all the fundamental facts of chemistry had been understood
in the sense that they could be reduced to the laws of atomic physics... Thus
the two sciences of physics and chemistry were amalgamated.
The fundamental problem of chemistry was not solved. But it was clear,
or nearly so, that it was a physical problem. The attraction of two atoms
should follow from the physcial laws prevailing within the atom and it should
not be necessary to introduce any extra chemical forces. Thus it can be said
physics and chemistry had come together and met in the face of a common
unsolved problem. This problem was quantum theory.
The valence structure of the chemical formula always plays a dominant
role, although it is accompanied by other structures. This provides the
physical justification of the formulae found and used by chemists since
the beginnings of chemistry.
Such an approach was particularly unappealing to London and not even
its success could convince him. Despite the fact that their joint paper was a
classic example of reductionism, London's subsequent work in quantum
chemistry contains timid expressions of a search for an alternative non-
reductionist approach. He seemed to be trying to find the fringes of a net he
had successfuliy woven in his school essays and, especially, in his doctoral
thesis in philosophy. It is ironic that his first important paper deviated so
far from his desire to view theories as wholes. But by the end of 1936,
when the correspondence with Heitler had ended, London was well on his
way to boosting his neglected program: having found the fringes, he was
beginning to weave a new net to accommodate the notion of a macroscopic
quantum phenomenon.
(203)
The Americans and the Germans: A necessary symbiosis.
Thomas Kuhn asked Mulliken whether there were different schools - a
Heitler-London school, a molecular orbital school - with some geographical
location, and whether in certain places one approach was being used and in
other places another approach, "so that people were somewhat past each
other?" Mulliken replied, "I do not know. The way I was thinking was
not in such terms as to notice things quite in that framework. I would say
there were some people who were stronger for one thing than for another,
but whether they were more abundant in one particular place I do not
know."
(204)
In response to the same questions, Wigner stated that he never
felt any opposition, since he realized from the very beginning that the two
approaches had different objectives. For example, the molecular orbital
method does not speak about the bond, but rather has molecular orbitals
that extend over the whole molecule: "This is too far away from the very
useful and very fruitful chemical concepts."
(205)
|
|

T.S. Kuhn
Photo and © from
here
|
The German theoretical physicist Friedrich Hund is ranked among the
founders of the molecular orbital approach. In 1928, just before sending an
important paper on the subject for publication, he received a preprint by
Mulliken who had essentially done the same calculations. Hund decided to
go ahead and publish his paper since "Mulliken's paper is rather American,
e.g., he proceeds by groping in an uncertain manner, where one can say
theoretically the cases for which a particular claim is valid."
(206)
Commenting on Mulliken's preprint, Van Vleck, an American, expressed the reverse
opinion: "The subject is indeed an interesting one, and a contribution from
you as a man thoroughly conversant with the experimental data is a valuable
supplement or rather complement to the rather abstract work of Hund.
(207)
Van Vleck expressed these differences again in a review article he wrote
in 1935 with Shennan. Anyone who looked for straightforward calculations
about chemical bonding from the basic postulates of quantum mechanics
was bound to be disappointed, they said. How then could it be said that we
have a quantum theory of valence? To give a satisfactory answer, one
"must adopt the mental attitude and procedure of the optimist rather than
the pessimist." The latter demands rigorous calculations from first principles
and does not allow questionable approximations or appeals to empirical
data. The optimist is content with approximate solutions of the wave equation
and "he appeals freely to experiment to determine constants, the direct
calculation of which would be too difficult.' The optimist believes that the
approximate calculations "give one an excellent 'steer' and a very good
idea of 'how things go,' permitting the systematization and understanding
of what would otherwise be a maze of experimental data codified by purely
empirical valence rules."
(208)
Physicists took several attitudes toward the status of quantum mechanics
in chemistry. Two were expressed at the meeting in 1931 of the British
Association for the Advancement of Science. Fowler observed that "the
chemical theory of valency is no longer an independent theory in a category
unrelated to general physical theory, but just a part - one of the most
gloriously beautiful parts - of a simple self-consistent whole, that is of non-
relativistic quantum mechanics. I have at least sufficient chemical appreciation
to say rather that quantum mechanics is glorified by this success than
that now 'there is some sense in valences,' which would be the attitude, I
think, of some of my friends."
(209)
Heisenberg thought that valence theory
would not have been so successful if chemical results about valency were
not already known: "I have tried to make the present quantum theory of
valence still more suspicious to the chemist than it already is. But we may
hope that after some time the theoretical physicists will be able to give a
more accurate explanation of what corresponds to the chemical valence."
(210)
Years later the changing role of theory in chemistry was stressed by
Coulson, a mathematician by training and the author of one of the standard
books on valence theory:
(211)
Valence Theory is about bonds: what they are, how many there are from each
atom, and why they are directed in the way they are. So fifty years of
valence theory really means fifty years of changing ideas about a chemical
bond. The first third of the period... was necessarily concerned with
identifying the electronic nature of the bond, and in escaping from the
thought forms
of the physicist, dominated by the center of symmetry in an isolated
atom, so
that the chemical notions of directional bonding and localization could be
developed... Has the chemical bond now done its job? Have we grown to
that degree of knowledge and that power of calculation that we do not need
it?... This is a tantalizing question. Chemistry is concerned to explain, to
give us insight and a sense of understanding. Its concepts operate at an
appropriate depth and are designed for the kind of explanation required and
given. If the level of inquiry deepens, as a result of our better
understanding,
then some of our older concepts 110 longer keep their relevance.... From its
very nature a bond is a statement about two electrons, so that if the behavior
of these two electrons is significantly dependent upon, or correlated with,
other electrons, our idea of a bond separate from, and independent of, other
bonds must be modified... Whither "something bigger" that should replace
the chemical bond, will come to US or not is a subject for a Symposium
bearing for its title: the changing role of chemical theory.
The genesis and development of quantum chemistry as an autonomous
subdiscipline owed much to scientists who successfully escaped "from the
thought forms of the physicist" by implicitly and explicitly taking on questions
about the role of theory and the methodological status of empirical
observations for theory building in chemistry.
References and notes
Kostas Gavroglu: Department of Physics, National Technical University, Zografu Campus, Athens 157 80, Greece.
Ana Simões: Department of Physics, University of Lisbon, Campo Grande, Ed. Cl, 1700, Lisboa, Portugal.
We thank T. Arabatzis, A. Assmus, T. Benfey, S.G. Brush, J.L. Heilbron, Mary Jo Nye,
S. Sigurdsson, and S. Schweber for their comments.
K.G. thanks A. Thackray for his hospitality at die Beckman Center for the History of Chemistry, where part of this work was done.
A.S. thanks the following institutions: the American Philosophical Society,
the Gulbenkian Foundation (Portugal), and FLAD (Portugal).
(1)
P.A.M. Dirac,
Quantum mechanics of many-electron systems,
Royal Society of London, Proceedings,
A123 (1929), 714-733, on 714.
(2)
G.N. Lewis, Valence and the structure of atoms
and molecules. (New York, 1923), 56 - 57;
cf. R.E. Kohler,
The origin of G.N. Lewis theory of the shared pair bond,
Historical Studies in the Physical Sciences,
3 (1971), 343 - 376, and
cf. R.E. Kohler,
The Lewis-Langmuir theory of valence and the chemical
community 1920 - 1928,"
Historical Studies in the Physical Sciences,
6 (1975), 431-468.
(3)
J.J. Thomson,
On the structure of the atom,
Philosophical Magazine, 26 (1913), 729 - 799, and
J.J. Thomson,
The forces between atoms and chemical affinity,
Philosophical Magazine, 27 (1914), 757-789.
(4)
W.C. Arsem,
A theory of valency and molecular structure,
Journal American Chemical Society, 36 (1914), 1655-1675.
(5)
A.L. Parson,
A magneton theory of the structure of the atom,
Smithsonian miscellaneous collections, 65 (1915), 1-80.
(6)
W. Kossel,
Über Molekülbildung als Frage des Atombaues,
Annalen der Physik, 49 (1916), 229-362.
(7)
Lewis (ref. 2), 56.
(8)
Ibid., preface.
(9)
Ibid., 74.
(10)
Lewis to Noyes, 11 Feb 1927 (G.N. Lewis Correspondence, The Bancroft Library, University of California at Berkeley).
(11)
J.J. Thomson,
Introduction to the session on the electronic theory of valency,
Faraday Society, Transactions, 19 (1923-24), 450.
(12)
N.V. Sidgwick,
The electronic theory of valency
(Oxford. 1927), preface.
(13)
E.C. Kemble, R.T. Birge, W.F. Colby, F.W. Loomis, and L. Page,
Molecular spectra in gases,
National Research Council, Bulletin, 11 (1926), 1-358.
(14)
A. Assmus,
Molecular structure and the genesis of the American quantum physics
community, 1916 - 1926,
Dissertation abstracts international, 52 (1991), 1057 - A;
A. Assmus,
The molecular tradition in early quantum theory,
Historical Studies in the Physical Sciences, 22 (1992), 209-23 I;
A. Assmus,
The Americanization of molecular physics,
Historical Studies in the Physical Sciences, 23 (1993), 1-33.
(15)
D.A. Ramsay and J. Hinze, eds.,
Selected papers of R.S. Mulliken
(Chicago, 1975).
This volume contains a complete list of Mulliken's publications.
An assessment of Mulliken's contributions to quantum chemistry is:
P.O. Löwdin and B. Pullman, eds.,
Molecular orbitals in chemistry, physics,
and biology: a tribute to R.S. Mulliken
(New York, 1964).
Mulliken assessed his own contribution in his
Life of a scientist:
an autobiographical account of the development of molecular orbital
theory with an introductory memoir by Friedrich Hund,
ed. B.J. Ransil (Berlin, 1989).
(16)
E.C. Kemble et al. (ref. 13), 238.
(17)
Arnold Sommerfeld,
Atomic structure and spectral lines,
trans. Henry L. Brose (New York, 1923);
Irving Langmuir,
The arrangement of electrons in atoms and molecules,
Journal American Chemical Society, 41 (1919), 868-934.
(18)
Mulliken to Birge, Jun 1925 (R.T.Birge Correspondence,
The Bancroft Library, University of California at Berkeley, Box 21).
(19)
R.T. Birge,
The energy levels of the carbon monoxide molecule,
Nature, 117 (1926), 229-230, and :
R.T. Birge,
The band spectra of carbon monoxide,
Physical Review, 28 (1926), 1157-1181; and also :
H. Sponer,
Anregunspotentiale der Bandenspektren des Stickstoffs,
Zeitschrift für Physik, 34 (1925), 622-633; and :
R. Mecke,
Zum Wesen der Dublettstruktur einer Klasse von Bandenspektren,
Naturwissenschaften, 13 (1925), 755-756,
(20)
R.T. Birge Correspondence, The Bancroft Library, Boxes 21, 31, and 32.
(21)
R.T. Birge,
The structure of molecules,
Nature, 117 (1926), 300-302, on 301.
(22)
R.S. Mulliken,
The electronic states of the helium molecule,
National Academy of Sciences, Proceedings, 12 (1926), 158 - 162.
(23)
R.S. Mulliken,
Systematic relations between electronic structure and band-spectrum structure in
diatomic molecules, I,
National Academy of Sciences, Proceedings, 12 (1926), 144 - 157.
In R.S. Mulliken Papers, University of Chicago Library, 81/16,
Molecular models and
Dynamics of molecular electrons,
there appear several sketches of molecular structures that Mulliken might
have drawn in formulating his postulates.
(24)
R.S. Mulliken, Systematic relations between electronic
structure and band-spectrum structure in diatomic molecules,
III: Molecule formation and molecular structure,
National Academy of Sciences, Proceedings, 12 (1926), 338 - 343.
(25)
F. Hund,
Zur Deutung einiger Erscheinungen in den Molekelspektren,
Zeitschrift für Physik, 36 (1926), 657 - 674.
(26)
Mulliken to Birge, 17 Oct 1926 (R.T.Birge Correspondence,
The Bancroft Library, University of California at Berkeley, Box 21).
(27)
R.S. Mulliken,
Electronic states. IV. Hund's theory; second positive nitrogen and swan bands;
alternate intensities,
Physical Review, 29 (1927), 637 - 649.
In the concluding chapter of ref. 13, Kemble showed how the recent developments
in quantum theory could be incorporated into the theory of band spectra, and
introduced Hund's theory to American physicists and chemists.
(28)
Mulliken, Life (ref. 15), 59.
(29)
Talk on 24 Feb 1928 (R.S. Mulliken Papers, University of Chicago Library, 72/14);
Mulliken to Van Vleck, 11 Apr 1928 (Archive for History of Quantum Physics, Reel 49).
(30)
Mulliken to Van Vleck, 21 May 1928
(Archive for History of Quantum Physics, Reel 49):
"Have you looked at the MSS
on electron quantum numbers that I sent to Tate a while ago? I am anxious to get
someone to find fault with it."
The answer followed in Van Vleck to Mulliken, 23 Jun 1928: "I am afraid
that I am unable to supply any 'severe criticism' such as you requested,
although
I read the paper through twice. The subject is indeed an interesting one, and a
contribution from you as a
man thoroughly conversant with the experimental data is a valuable supplement or
rather complement to the rather abstract work of Hund."
(31)
R.S. Mulliken,
The assignment of quantum numbers for electrons in molecules,
Physical Review, 32 (1928), 186 - 222, on 190.
(32)
R.S. Mulliken,
Systematic relations (ref. 23).
In the old quantum theory such a drastic
change could only be justified when the molecule was formed by violent means,
such as collision or light absorption.
(33)
R.S. Mulliken Papers, University of Chicago Library, 80/17.
(34)
Interview with Mulliken (Archive for History of Quantum Physics).
(35)
R.S. Mulliken, Quantum numbers
(ref. 31), 191 - 192.
(36)
Ibid., 194.
(37)
Ibid., 196.
(38)
Ibid., 201.
(39)
J.C. Slater, Robert Mulliken of Newburyport,
in Löwdin and Pullman (ref. 15), 19.
(40)
Gale to Mulliken, 6 and 22 Mar and 6 Apr 1928; draft of letter from Mulliken
to Gale, 24 Mar 1928 (R.S. Mulliken Papers, University of Chicago Library, 107/2).
(41)
R.S. Mulliken, Life (ref. 15), 67, and
The interpretation of band spectra,
R.S. Mulliken Papers, University of Chicago Library, 2 (1930), 60 - 115,
3 (1931), 89 - 155, and 4 (1932), 1 - 86.
(42)
J.H. Van Vleck and A. Sherman,
The quantum theory of valence,
R.S. Mulliken Papers, University of Chicago Library, 7 (1935), 167 - 228,
on 175.
(43)
R.S. Mulliken Papers, University of Chicago Library, 82/8 - 11.
(44)
A complete bibliography of Pauling's papers from 1920 to 1990, compiled by
Z.S. Hermann and D.B. Munro, is contained in C.S. Mead, ed.,
The Pauling catalog: Ava Helen and Linus Pauling papers,
Kerr Library Special Collections, Oregon State University (Corvallis, 1991);
cf. A. Rich and N. Davidson, eds., Structural chemistry and molecular biology:
a volume dedicated to Linus Pauling by his students, colleagues and friends
(San Francisco, 1968).
(45)
L. Pauling,
The prediction of the relative stabilities of isosteric isomeric ions and
molecules,
Journal American Chemical Society, 48 (1926), 641-651.
(46)
L. Pauling,
The dynamic model of the chemical bond and its
application to the structure of benzene,
Journal American Chemical Society, 48 (1926), 1132 - 1143.
In a seminar in 1925 Pauling discussed the idea of
binuclear orbits (Ava Helen and Linus Pauling Papers, Kerr Library Special
Collections, Oregon State University, Box 242, Popular scientific
lectures 1925 - 35,
Modern theories of valence and chemical
combination).
(47)
Pauling to Noyes, 25 Apr, 22 May, and 22 Nov 1926 (Ava Helen and Linus Pauling
Papers,Kerr Library Special Collections, Oregon State University, Box 71).
(48)
Interview with Pauling (Archive for History of Quantum Physics).
(49)
Pauling to Noyes, 22 May 1926 (Ava Helen and Linus Pauling Papers, Kerr Library Special Collections, Oregon State University, Box 71);
L. Pauling, The quantum theory of the
dielectric constant of hydrogen chloride and similar gases,
National Academy of Sciences, Proceedings, 12 (1926), 32 - 35;
also in
Physical Review, 27 (1926), 568 - 577.
(50)
Pauling to Noyes, 12 Jul 1926 (Ava Helen and Linus Pauling Papers,
Kerr Library Special Collections, Oregon State University, Box 71).
Pauling presented the work in a meeting on magnetism organized by Debye in
Zürich and published it soon thereafter as :
L. Pauling, The influence of a magnetic field on the
dielectric constant of a diatomic dipole gas,
Physical Review, 29 (1927), 145 - 160.
(51)
L. Pauling,
Fifty years of progress in structural chemistry and molecular
biology,
Daedalus, 99 (1970), 988 - 1014, on 993.
(52)
Pauling to Noyes, 12 Jul, 22 Nov, and 17 Dec 1926
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 71, Noyes correspondence, 1921 - 38).
(53)
L. Pauling,
The electron affinity of hydrogen and the second ionization potential of
lithium,
Physical Review, 29 (1927), 285 - 291.
(54)
L. Pauling,
The theoretical prediction of the physical properties of many-electron atoms
and ions,
Royal Society of London, Proceedings, A114 (1927), 181 - 211.
The letter written by Sommerfeld is in Ava Helen and Linus Pauling Papers,
Kerr Library Special Collections, Oregon State University, Box 97.
(55)
Interview with Pauling (Archive for History of Quantum Physics).
(56)
R.S. Mulliken,
Molecular scientists and molecular science: some reminiscences,
Journal of Chemical Physics, 43 (1965), S2 - S11, on S7.
(57)
Interview with Heitler (Archive for History of Quantum Physics).
(58)
W. Heitler and F. London,
Wechselwirkung meutraler Atome und homöpolare Bindung nach der
Quantenmechanik,
Zeitschrift für Physik, 44 (1927), 455 - 472, on 472.
One of the main drawbacks of their approach was omitting the ionic terms.
Cf. Van Vleck and Sherman (ref. 42).
(59)
Dictionary of scientific biography, s.v. "Schrödinger."
(60)
Sir Brian Pippard, private communication to KG.
(61)
Interview with Heitler (Archive for History of Quantum Physics).
(62)
J.L. Hellbron,
The origins of the exclusion principle,
Historical Studies in the Physical Sciences, 13 (1983), 261 - 3 10.
See also B.L. van der Waerden,
Exclusion principle and spin,
in: M. Fierz and V.F. Weisskopf,
eds., Theoretical physics in the twentieth century, a memorial volume to
W. Pauli (New York, 1960), and
H. Margenau,
The exclusion principle and its philosophical tradition,
Philosophy of Science, 11 (1944), 187 - 208.
(63)
H. Weyl,
Encomium,
Science, 103 (22 Feb 1946), 216 - 218;
W. Pauli,
Remarks on the history of the exclusion principle,
Science, 103 (22 Feb 1946), 213 - 215.
(64)
S. Goudsmit and G.E. Uhlenbeck,
Ersetzung der Hypothese von unmechanischen
Zwang durch eine Forderung bezüglich des inneren VerhaItens jedes
einzelnen Elektrons,
Naturwissenschaften, 13 (1925), 953 - 954, and
Spinning electrons and the structure of
spectra,
Nature, 107 (1926), 264 - 265;
W. Pauli,
Zur Quantenmechanik des magnetischen Elektrons,
Zeitschrift für Physik, 43 (1927), 601 - 623;
C.G. Darwin,
The electron as a vector wave,
Royal Society of London, Proceedings, 116 (1927), 227 - 253;
P.A.M. Dirac,
Quantum theory of the electron,
Royal Society of London, Proceedings, 117 (1928), 610 - 624.
(65)
Interview with Heitler (Archive for History of Quantum Physics).
(66)
F. London,
Quantentheorie und chemische Bindung,
in : Quantentheorie und Chemie (Leipzig, 1928), 59 - 84, on 71;
cf. Heitler and London (ref. 58), 456.
(67)
Van Vleck,
Spin, the great indicator of valence behavior,
Pure and Applied Chemistry, 24 (1970), 235 - 255, on 240.
(68)
Heitler to London, Sep 1927 (Fritz London Archives, Duke University).
(69)
Ibid.
(70)
Interview with E. Wigner (Archive for History of Quantum Physics).
(71)
Interview with Heitler (Archive for History of Quantum Physics).
(72)
Heitler to London, 7 Dec 1927 (Fritz London Archives, Duke University).
(73)
Interview with Heit!er (Archive for History of Quantum Physics).
(74)
Hartree to London, 16 Sep 1928 (Fritz London Archives, Duke University).
(75)
W. Heitler and G. Rumer,
Quantentheorie der Chemischen Bindung in Mehratomige Molecüle,
Zeitschrift für Physik, 68 (1931), 12 - 3 I.
(76)
Interview with Heitler (Archive for History of Quantum Physics).
(77)
F. London,
Zur Quantentheorie der homöopolaren Valenzzahlen,
Zeitschrift für Physik, 46 (1928), 455 - 467, on 459.
(78)
London (ref. 66), 60.
(79)
F. London,
Zur Quantenmechanik der homoopolaren Valenzchemie,
Zeitschrift für Physik, 50 (1928), 24 - 51, on 48.
(80)
F. London,
Die Bedentung der Quantentheorie für die Chemie
(Planck Festschrift),
Naturwissenschaften, 17 (1929), 516 - 529;
cf. F. London, Valenzzahlen (ref. 77),
Valenzchemie (ref. 79), and
Quantentheorie und Chemische Bindung
(ref. 66).
(81)
L. Pauling,
The application of the quantum mechanics to the structure of the hydrogen
molecule,
Chemical Reviews, 5 (1928), 173 - 213, on 174.
(82)
J.H. Van Vleck,
The new quantum mechanics,
Chemical Reviews, 5 (1928), 467 - 507, on 506.
(83)
Louis de Broglie,
Opening address to the First International Congress of Quantum
Chemistry at Menton, France, July 4 - 10, 1973, in :
R. Daudel and B. Pullman, eds.,
The world of quantum chemistry (Dordrecht, 1974);
Max von Laue,
History of Physics (New York, 1950), 139.
(84)
L. Pauling and E.B. Wilson,
Introduction to quantum mechanics with applications to
chemistry, (New York, 1935), 340.
(85)
W. Heisenberg,
Contribution to the discussion on the structure of simple molecules,
in : Chemistry at the centenary (1931) meeting of the British Association
for die Advancement of Science (Cambridge, 1932), 247 - 248, on 247.
(86)
A.D. Buckingham,
Quantum chemistry, in :
C.W. Kilmister, ed., Schrödinger -
centenary celebration of a polymath (Cambridge, 1987), 112 - 117;
quoting McCrea, "How quantum physics came to England,
New Scientist, 17 (Oct 1985), 58 - 60.
(87)
Interview with Wigner (Archive for History of Quantum Physics).
(88)
Weyl,
Group theory and quantum mechanics
(New York, 1931), 342, 346, 370.
(89)
Cf. Mulliken's article,
Band spectra and chemistry,
Chemical Reviews, 6 (1929), 503 - 545,
specifically for the chemists.
(90)
L. Pauling,
The application of the quantum mechanics to the structure of the hydrogen
molecule and the hydrogen molecule-ion and to related problems,
Chemical Reviews, 5 (1928), 173 - 213.
(91)
Private communication to K.G.
(92)
Van Vleck (ref. 82).
(93)
J.H. Van Vleck,
The group relation between the Mulliken and Slater-Pauling theories of
valence,
Journal of Chemical Physics, 3 (1935), 803 - 806.
(94)
Van Vleck and Sherman (ref. 42), 173.
(95)
Van Vleck (ref. 82), 500, 503.
(96)
G.L. Clark,
Introductory remarks in the Symposium on atomic structure and
valence,
Chemical Reviews, 5 (1928), 361 - 364, on 362.
(97)
W.H. Rodebush,
The electron theory of valence,
Chemical Reviews, 5 (1928), 509 - 53 I, on 511, 513.
(98)
H.S. Fry,
A pragmatic system of notation for electronic valence conceptions in chemical
formulas,
Chemical Reviews, 5 (1928), 557 - 568, on 558 - 559.
Emphasis added.
(99)
F. Hund,
Chemical binding,
Faraday Society, Transactions, 25 (1929), 645 - 647.
(100)
J.E. Lennard-Jones,
The electronic structure of some diatomic molecules,
Faraday Society, Transactions, 25 (1929), 665 - 686;
W. Heitler,
Zur Quantentheorie der Valenz,
Naturwissenschaften (5 Jul 1929), 546 - 547.
(101)
Pauling to Van Vleck, 9 Aug 1927 (Ava Helen and Linus Pauling Papers,
Kerr Library Special Collections, Oregon State University, Box 113,
Van Vleck Correspondence).
(102)
Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 210, Linus Pauling Calculations and Manuscripts,
vol. III, 1926 - 27.
(103)
Pauling (ref. 46).
(104)
0. Burrau,
Berechnung des Energiewertes des Wasserstoffmolekel-Ions
(H2+, Videnskabernes Selskab,
Matematisk-Fysiske Meddelelser, 7 (1927).
(105)
Archive for History of Quantum Physics and Pauling,
Work on molecular orbitals,
1926 (ref. 102).
(106)
Interview with Pauling (Archive for History of Quantum Physics).
(107)
E.U. Condon,
Wave mechanics and the normal state of the hydrogen molecule,
National Academy of Sciences, Proceedings, 13 (1927), 466 - 470.
(108)
Interview with Pauling (Archive for History of Quantum Physics).
(109)
Interview with Pauling (Archive for History of Quantum Physics);
Pauling to Van Vleck (ref. 101);
L. Pauling,
The combination of two hydrogen atoms,
Zürich, 21 Jun 1927 and
He spectrum or H2.
Two helium atoms,
Zürich, Jun-Jul 1927 (ref. 102).
(110)
Pauling to Van Vleck (ref. 101).
(111)
L. Pauling,
The shared-electron chemical bond,
National Academy of Sciences, Proceedings, 14 (1928), 359 - 362.
(112)
Ref. 105.
(113)
Archive for History of Quantum Physics and Pauling,
He spectrum.
(ref. 109).
(114)
Interview with Pauling (Archive for History of Quantum Physics).
(115)
Ibid. He learned group theory with Bateman at Cal Tech.
(116)
Archive for History of Quantum Physics, and:
Pauling,
Group theory and chemical combination.
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University , Box 209, Linus Pauling
Notes and Calculations, vol. II, 1923 - 29).
This includes his first discussion of hybridization
and comments on London,
Valenzzahlen
(ref.77).
(117)
Pauling (ref. 111).
(118)
Pauling (ref. 111), on 361.
(119)
Private communication to A.S.
(120)
Birge to Mulliken, 18 Apr 1931 (R.T.Birge Correspondence,
The Bancroft Library, University of California at Berkeley).
(121)
Pauling to Lamb, 11 Feb 1931 (Ava Helen and Linus Pauling Papers, Kerr Library
Special Collections, Oregon State University, Box 168, American Chemical Society:
Correspondence 1925 - 44).
In the interview in Archive for History of Quantum Physics, Pauling
referred to the frequent delays in publication in the Journal American Chemical Society, but especially to the problems he had with the editor and referees due to the
theoretical nature of his papers. In 1929 he published a paper on "The principles determining the structure of complex ionic crystals," which had to be reduced by half to comply with the editor's request.
(122)
L. Pauling,
Quantum mechanics and the chemical bond,
Physical Review, 37 (1931), 1185 - 1186;
J.C. Slater,
Directed valence in polyatomic molecules,
Physical Review, 37 (1931), 481 - 489;
S.S. Schweber,
The young Clark Slater and the development of quantum chemistry,
Historical Studies in the Physical Sciences, 20:2(1990), 339-406.
(123)
Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University , Box 212,
Linus Pauling Berkeley Lecture: Quantum Mechanics 1929 - 33, introduction to first
lecture on The nature of the chemical
bond.
(124)
L. Pauling,
The nature of the chemical bond. Application of results obtained from the
quantum mechanics and from a theory of paramagnetic susceptibility to the
structure of molecules,
Journal American Chemical Society, 53 (1931), 1367 - 1400, on 1367.
(125)
Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 212,
Linus Pauling Berkeley Lectures: Quantum Mechanics 1929 - 33,
conclusion to the lectures on
The nature of the chemical bond.
Pauling stated that "the rules have worked out
so well that even if there were no such justification they couid be
accepted on an empimical basis.
(126)
Interview with Pauling (Archive for History of Quantum Physics).
(127)
Ref. 89. In a report on work in progress prepared in 1930, Mulliken stressed
the importance of molecular spectra for both physicists and chemists:
"[Band spectra's] methods of analysis are those of physics both in the
experimental and theoretical steps of the process; the
application of results is of special interest to chemistry.
In other words this field of molecular spectroscopy is of interest to
both physicists and chemists, although it is pure science from the
standpoint of the physicist, but more nearly applied science from that
of the chemist" (R.S. Mulliken Papers,
University of Chicago Library, 84/10).
(128)
Birge to Mulliken, I Feb 1930 (R.T.Birge Correspondence, The Bancroft Library,
University of California at Berkeley, Box 33).
(129)
Mulliken to Birge, 26 Mar 1931 (R.T.Birge Correspondence, The Bancroft Library,
University of California at Berkeley, 21).
(130)
Birge to Mulliken, 17 Mar 1928 (R.T.Birge Correspondence, The Bancroft Library,
University of California at Berkeley, 32).
(131)
Mulliken to Birge, Leipzig [1930] (R.T.Birge Correspondence, The Bancroft Library,
University of California at Berkeley, 21).
(132)
R.S. Mulliken (ref. 128),
Bonding power of electrons and theory of valence,
Chemical Reviews, 9 (1931), 347 - 388, on 347, 384, 369.
(133)
Ibid., 350, 351.
(134)
Ibid., 354.
(135)
Ibid., 357, 360.
(136)
Ibid., 383.
(137)
Ibid., 357, 359. Mulliken was the first to use the analogy of spin as an indicator
of valence stressed in Van Vleck (ref. 67).
(138)
Ibid., 384 - 385.
(139)
Ibid., 369.
(140)
The later parts of
The nature of the chemical bond, were:
II. The one-electon bond and
the three-electron bond,
Journal American Chemical Society, 53 (1931), 3225 - 3237;
III. The transition from one extreme bond type to
another,
Journal American Chemical Society, 54 (1932), 988 - 1003;
IV. The energy of single bonds and the relative
electronegativity of atoms,
Journal American Chemical Society, 54 (1932), 3570 - 3582;
V. The quantum mechanical calculation
of the resonance
energy of benzene and naphthalene and the
hydrocarbon free radicals,
Journal of Chemical Physics, 1 (1933), 362 - 374, with G. Wheland;
VI. The calculation from thermochemical
data of the energies of resonance of molecules
among several electronic
structures,
Journal of Chemical Physics, 1 (1933), 606 - 617,
with J. Sherman;
VII. The calculation of resonance energy
in conjugated systems,
Journal of Chemical Physics, 1 (1933), 679 - 686.
(141)
L. Pauling,
Recent work on the structure of molecules,
talk given to the Southern Section of the American Chemical Society,
1936 (Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University , Box 242,
Popular Scientific Lectures 1925 - 55).
(142)
L. Pauling, Resonance and organic chemistry,
1941 (ibid.).
(143)
L. Pauling,
The theory of valence and of the structure of atoms, molecules, and
crystals,
1957 (Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 243, Popular Scientific Lectures 1956 - 65).
(144)
According to Pauling, his Quantum Mechanics holds the record of continuous
publication without modifications by its publisher McGraw-Hill
(New York, 1935 - 85).
(145)
Lewis to Pauling, 25 Aug 1939 (Ava Helen and Linus Pauling Papers, Kerr Library
Special Collections, Oregon State University, Box 58).
(146)
The draft chapters are in Ava Helen and Linus Pauling Papers, Kerr Library
Special Collections, Oregon State University, Box 372.
(147)
Pauling to Wheland, 30 Oct 1936, 13 and 30 Mar 1937, and 28 Jui 1937; Wheland to
Pauling. 29 Nov 1936 (Ava Helen and Linus Pauling Papers, Kerr Library
Special Collections, Oregon State University, Box 115).
(148)
Pauling to Wheland, 1 Dec 1944 and 27 Jun 1945; Wheland to Pauling, 6 Dec 1944
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 115).
(149)
The nature of the chemical bond 1932 - 59
(Ava Helen and Linus Pauling Papers,
Kerr Library Special Collections, Oregon State University, Box 399).
(150)
L. Pauling,
The nature of the chemical bond and the structure of molecules and crystals.
(New York, 1939), 12.
(151)
Interview with Pauling (Archive for History of Quantum Physics):
"I take a sort of Bridgmanian attitude toward [questions of interpretation of
quantum mechanics]. Bridgman with his ideas about operational
significance of everything would say that a question that does not have
operational significance... is meaningless."
(152)
Pauling to Wheland, 24 May 1943;
Wheland to Pauling, 17 May 1943, 6 Dec 1944, and 21 Apr 1954
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 115);
G.W. Wheland,
The theory of resonance and its applications to organic chemistry.
(New York, 1944).
(153)
Wheland to Pauling, 20 Jan 1956
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 115).
(154)
Pauling to Wheland, 26 Jan and 8 Feb 1956
(Ava Helen and Linus Pauling Papers, Kerr Library Special Collections,
Oregon State University, Box 115).
(155)
Wheland to Pauling, 4 Feb 1956 (PF. Box 115).
(156)
Ref. 154.
(157)
L. Pauling,
Modern structural chemistry,
Nobel lecture, December 11, 1954,
in Nobel lectures in chemistry 1942 - 1962 (Amsterdam, 1964), 134 - 148.
(158)
L. Pauling,
The nature of the theory of resonance,
in Sir Alexander Todd, ed., Perspectives in organic chemistry, dedicated to
Sir Robert Robinson (New York, 1956); and
The nature of the chemical bond and the structure of molecules and
crystals.
(3rd edn., New York, 1967).
(159)
L. Pauling,
The nature of theory of resonance.
(ref. 158), 6 - 7, and
The nature of the chemical bond.
(ref. 158), 219 - 220.
(160)
J.R. Platt,
1966 Nobel Laureate in chemistry: Robert S. Mulliken,
Science, 154 (1966), 745 - 747, on 746.
(161)
R.S. Mulliken,
Electronic structure of polyatomic molecules and valence,
Physical Review, 40 (1932), 55 - 62.
(162)
Mulliken to Van Vleck, 28 Feb and 28 Mar 1932
(Archive for History of Quantum Physics, Reel 49).
(163)
F. Hund,
Zur Frage der chemischen Bindung,
Zeitschrift für Physik, 73 (1931), 1 - 30.
(164)
R.S. Mulliken,
Electronic structures of polyatomic molecules and valence. II. General
considerations,
Physical Review, 41 (1932), 49 - 71, on 51.
(165)
Ibid., 52.
(166)
Ibid., 60.
(167)
Mulliken to Van Vleck, 16 Apr 1935
(American Institute of Physics, Van Vleck Papers, 38/47).
(168)
R.S. Mulliken,
Electronic structures of polyatomic molecules and valence. VI. On the
method of molecular orbitals,
Journal of Chemical Physics, 3 (1935), 375 - 378, on 376.
(169)
Mulliken (ref. 168), 378. He made the same point in a letter to Van Vleck of 1 Jul
1935(?) (Archive for History of Quantum Physics, Reel 49).
(170)
R.S. Mulliken,
Electron states and the structure of molecules
(R.S. Mulliken Papers, University of Chicago Library, 74/2).
(171)
R.S. Mulliken, Selected papers (ref. 15), on 13,
an excerpt from Mulliken's speech on receiving Lewis' award,
What are the electrons really doing in molecules,
Vortex, 21 (1960), 1 - 5.
(172)
R.S. Mulliken,
Electronic structure of molecules,
talk at the Chicago meeting of the National Academy of Sciences(?),
1937(?), (R.S. Mulliken Papers, University of Chicago Library, 74/6).
(173)
Private communication to A.S.
(174)
Heitler to London, 4 Nov 1935 (Fritz London Archives, Duke University).
(175)
Heitler to London, 12 Nov 1935 (Fritz London Archives, Duke University).
(176)
Ibid.
(177)
Ibid.
(178)
London to Heitler, Oct or Nov 1935 (Fritz London Archives, Duke University).
(179)
London to Heitler, 17 Nov 1935 (Fritz London Archives, Duke University);
Heitler to London, 22 Nov 1935 (Fritz London Archives, Duke University).
(180)
London to Heitler, Nov 1935 (Fritz London Archives, Duke University).
(181)
R.S. Mulliken,
Interpretation of band spectra. III.
(ref. 41), 30.
(182)
R.S. Mulliken,
Quantum numbers I (ref. 31), 189, and
The assignment of quantum numbers for electrons in molecules.
II. the correlation of molecular and atomic electron states,
Physical Review, 32 (1928), 761 - 772.
(183)
R.S. Mulliken,
The assignment of quantum numbers for electrons in molecules.
III. Diatomic hydrides,
Physical Review, 33 (1929), 731 - 747.
(184)
R.S. Mulliken,
Electronic structures of polyatomic molecules and valence.
V. Molecular RXn ,
Journal of Chemical Physics, 1 (1933), 492 - 503.
(185)
R.S. Mulliken,
Spectroscopy, molecular orbitals and chemical bonding,
Science, 157 (7 Jul 1967), 17.
(186)
R. Kronig,
The optical basis of valency. (Cambridge, 1935).
(187)
Ibid.
(188)
G.W. Wheland,
The quantum mechanics of unsaturated and aromatic molecules:
A comparison of two methods of treatment,
Journal of Chemical Physics, 2 (1934), 474 - 481.
(189)
Heitler to London, beginning of Dec 1935 (Fritz London Archives, Duke University).
(190)
Heitler to London, 13 Dec 1935 (Fritz London Archives, Duke University).
(191)
Ibid.
(192)
Heitler to London, 6 Feb 1936 (Fritz London Archives, Duke University).
(193)
Heitler to London, 7 Oct 1936 (Fritz London Archives, Duke University).
(194)
London to Heitler, Nov(?) 1936 (Fritz London Archives, Duke University).
(195)
London to Born, I Oct 1936 (Fritz London Archives, Duke University).
(196)
Born to London, 10 Oct 1936 (Fritz London Archives, Duke University).
(197)
Heitler to London, 7 Oct 1936 (Fritz London Archives, Duke University).
(198)
W. Heitler,
Elementary wave mechanics with applications to chemistry.
(Oxford, 1956); first published in 1945 as Elementary vvave mechanics.
(199)
W. Heitler and G. Poschl,
Ground states of C2 and 02 and the theory
of valence.
Nature (2 Jun 1934), 833-834.
(200)
Cf., M.J. Nye,
Physics and chemistry: Commensurate and incommensurate sciences?,
in Nye and J. Richards, eds.,
The invention of physical science.
(Dordrecht, 1992), 205 - 224.
(201)
W. Heitler,
Wave mechanics. (ref. 198), on 190.
(202)
The following quotes come from, respectively,
W. Heitler,
Quantum chemistry: the early period,
International Journal of Quantum Chemistry, 1 (1967), 13 - 36,
on 14, 35, 13, and
W. Heitler,
The theory of the chemical bond,
Archiv für Physik, 10 (1955), 145 - 156, on 156.
(203)
K. Gavroglu,
Fritz London: A scientific biography.
(Cambridge, 1994).
(204)
Interview with Mulliken (Archive for History of Quantum Physics).
(205)
Interview with Wigner (Archive for History of Quantum Physics).
(206)
Hund to London, 13 Jul 1928 (Fritz London Archives, Duke University).
(207)
Van Vleck to Mulliken, 23 Jun 1928
(Archive for History of Quantum Physics, Reel 49).
(208)
Van Vleck and Sherman (ref. 42), 168 - 169.
(209)
R.H. Fowler,
A report on homopolar valency and its quantum mechanical
interpretation, in :
Chemistry at the centenary (1931) meeting of the British Association for the
Advancement of Science.
(Cambridge, 1932), 226 - 246, on 226.
(210)
W. Heisenberg,
Contributions to the discussion on the structure of simple molecules,
ibid., 247 - 248.
(211)
C.A. Coulson,
Recent developments in valence theory,
Pure and Applied Chemistry, 24 (1970), 257 - 287, on 259, 287.
Emphasis not in original.
















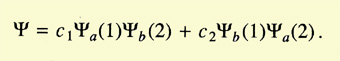
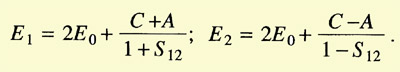
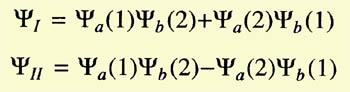

 and
and
 )
signified attraction:
(69)
)
signified attraction:
(69)
 and
and
 are always attracted in a homopolar manner. We can, then, eat
Chemistry with a spoon.
are always attracted in a homopolar manner. We can, then, eat
Chemistry with a spoon. (C has to be excited from its ground state in order to be in
(C has to be excited from its ground state in order to be in
 which agrees with experience). Four different "cells" in the L-
shell exist for four electrons combined antisymmetrically. The four
hydrogen atoms accordingly would be
which agrees with experience). Four different "cells" in the L-
shell exist for four electrons combined antisymmetrically. The four
hydrogen atoms accordingly would be
 .
Methane could therefore be
reduced to a simple formula: the four hydrogen atoms are attracted in a
homopolar manner to the carbon atom, without, however, any repulsion
among them. The tetrahedron resulted. It was all very promising: "The long
story for the foundational aspects of this matter, could give us, I believe, a
series of very simple and equally interesting observations, if it were
possible to approximate better the whole damn thing."
(72)
.
Methane could therefore be
reduced to a simple formula: the four hydrogen atoms are attracted in a
homopolar manner to the carbon atom, without, however, any repulsion
among them. The tetrahedron resulted. It was all very promising: "The long
story for the foundational aspects of this matter, could give us, I believe, a
series of very simple and equally interesting observations, if it were
possible to approximate better the whole damn thing."
(72)